Abstract
2485
Introduction: [18F]3F4AP is a radiofluorinated derivative of the multiple sclerosis drug 4-aminopyridine. [18F]3F4AP binds to K+ channels of demyelinated axons and has shown high sensitivity towards demyelinated lesions in rodent models of demyelination as well as to a focal traumatic brain injury in a rhesus macaque. We recently advanced this tracer to human studies through an IND and reported whole-body imaging and dosimetry estimation. Here we describe the first brain imaging results with [18F]3F4AP in healthy volunteers.
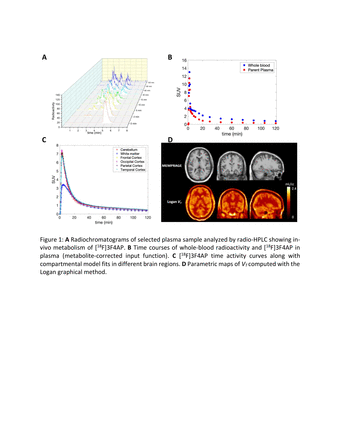
Methods: Three healthy volunteers (2 females and 1 male) were scanned on a GE Discovery MI PET/CT scanner and underwent a 120 min dynamic PET acquisition following a bolus injection of [18F]3F4AP (mean injected activity: 7.74 mCi, range: 7.64-7.88 mCi). Arterial blood samples were collected throughout the duration of the scan to determine radioactivity concentration in whole blood and plasma. Radiometabolite analysis was performed on a subset of plasma samples using a radio-HPLC system to derive individual metabolite-corrected arterial input functions. Participants also underwent a structural MEMPRAGE for anatomical information. Dynamic PET images were registered to the Montreal Neurological Institute (MNI) template space using the subject’s structural MRI to allow delineation of regions of interest for extraction of time activity curves. Various compartment models as well as the Logan graphical method with arterial plasma input function were tested for estimation of the total volume of distribution VT. Parametric maps of VT were obtained by applying the Logan graphical method at the voxel level.
Results: Whole-blood and total plasma radioactivity time courses were consistent across subjects. Whole-blood to plasma ratio stabilized to 0.88 ± 0.02 after 15 min (range: 0.84-0.93). Percent parent in plasma (%PP) time course was best fitted by an inverted Hill function. In contrast to preclinical studies performed in non-human primates where [18F]3F4AP was found to undergo very little in vivo degradation (>90% parent remaining after 2h post injection), in human subjects radio-HPLC analysis revealed relatively rapid in vivo metabolism with on average 31% (range 27-33%) of radioactivity attributable to the parent compound at 60 min post tracer injection. In the brain, [18F]3F4AP time activity curves peaked quickly (2-4 SUV in white matter and 5-7 SUV in gray matter at ~3 min) and was followed by a fast washout in all brain regions with little heterogeneity across cortical regions and white matter after 20 min. Regional brain time activity curves (TACs) were best fitted by a one-tissue (1T) compartment model and provided stable VT estimates. Quantitative analysis demonstrated a large range of K1 values (rate of transfer from plasma to tissue) across brain regions (0.24 mL/min/cc in white matter to 0.76 mL/min/cc in putamen) confirming a fast brain penetration and suggesting a high extraction fraction from vascular compartment to brain tissue. Regional total volume of distribution (VT) in gray matter ranged from 1.52-1.69 mL.cm-3 and 1.22-1.29 mL.cm-3 in white matter.
Conclusions: We report results from the first human brain imaging study with [18F]3F4AP. [18F]3F4AP was found to be highly brain permeable with fast clearance kinetics. [18F]3F4AP was found to undergo metabolism in humans faster than in monkeys. Differences in rate of peripheral metabolism observed across species may be due to the effect of anesthesia and is currently under investigation in rodents and in vitro (Abstract #865). Although late SUV images showed little heterogeneity across brain regions, VT images showed greater higher values in cortical areas compared to white matter, consistent with higher expression and accessibility of Kv channels in those regions. Future studies will include scans in patients with demyelinated disease such as multiple sclerosis.
Funding support: R01NS114066; S10OD018035; P41EB022544