Abstract
2445
Introduction: Exposure of sensitive tissues to ionizing radiation is an inherent risk and dose-limiting in radiopharmaceutical therapies. Developing radiation countermeasures (RC) that protect radiosensitive organs is urgently needed to reduce toxicity and improve patient outcomes. Herein, we showed that <u>extracellular vesicles (CRX EVs) derived from TLR-4 stimulated mesenchymal stromal cells home to and protect radiosensitive organs in response to lethal systemic irradiation. </u>
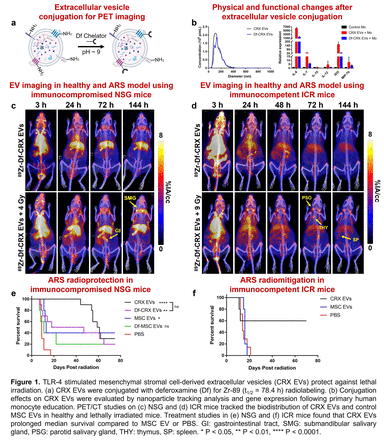
Methods: EVs were isolated by sequential centrifugation from primary bone marrow-derived MSCs stimulated with a TLR-4 receptor agonist (CRX-527; CRX EVs) or unstimulated as a control (MSC EVs). Effects of conjugating EVs with deferoxamine (Df; Df-CRX EVs) for Zr-89 radiolabeling (t1/2 = 78.4 h) were evaluated physically and functionally using nanoparticle tracking analysis to compare size and gene expression measuring their ability to polarize primary human monocytes into a reparative phenotype. Lethal systemic radiation toxicity models were established using whole-body irradiation (Xstrahl CIX3) of NSG (4 Gy) and ICR (9 Gy) mice. Longitudinal PET studies of intravenously injected radiolabeled EVs (89Zr-Df-CRX EVs or 89Zr-Df-MSC EVs, ~6.7 MBq), acquired using an Inveon microPET/CT and reconstructed using an OSEM3D/MAP algorithm, compared the biodistribution of CRX EVs and MSC EVs in immunocompromised NSG mice and immunocompetent ICR mice with and without lethal irradiation. EV biodistribution was quantified via volume-of-interest analysis using Inveon Research Workstation and expressed as percent injected activity per cubic centimeter (%IA/cc). After the last scanning time point at 144 h post-injection (p.i.), imaging mice were euthanized, and the major organs were collected for ex vivo quantification. Protection against lethal systemic irradiation by administering CRX EVs and MSC EVs either 4 h (NSG mice) or 24 h (ICR mice) following irradiation was evaluated by overall survival.
Results: Df conjugation did not alter the average EVs diameter (116 nm vs. 118 nm) but did moderately decrease their ability to polarize monocytes into a reparative phenotype. Longitudinal PET imaging studies found that CRX EVs and MSC EVs had comparable in vivo biodistribution, with excellent blood circulation (half-life ~12 h) and relatively low lung, liver, and spleen (excluding NSG mice) uptake. In irradiated NSG mice, CRX EVs and MSC EVs homed to the radiosensitive gastrointestinal tract at 72 h p.i. (CRX EVs: 4.4 ± 0.7 %IA/cc vs. 2.1 ± 0.2 %IA/cc, p = 0.004; MSC EVs: 4.9 ± 0.9 %IA/cc vs. 2.3 ± 0.3 %IA/cc, p = 0.009) and upward trends were observed for ex vivo submandibular salivary gland uptake at 144 h (CRX EVs: 8.9 ± 1.9 %IA/g vs. 5.3 ± 0.6 %IA/cc, p = 0.069; MSC EVs: 9.0 ± 2.6 %IA/cc vs. 5.4 ± 1.3 %IA/cc, p = 0.056) compared to healthy mice. Similarly, lethally irradiated ICR mice showed uptake increased EV uptake in the parotid salivary gland, thymus, and spleen. Treating lethally irradiated NSG mice with CRX EVs (5x109) extended median survival time to 60 d compared to MSC EVs (13 d) or PBS (9 d). Notably, conjugated CRX EVs used for PET retained therapeutic efficacy in the NSG model with a median survival of 32.5 d. Treatement with CRX EVs also prolonged survival in lethally irradiated ICR mice (median survival was not achieved at 70 d) compared to MSC EVs (16 d) or PBS (14 d).
Conclusions: The biodistribution of MSC-derived EVs, which was not altered by TLR-4 stimulation or Df conjugation, was marked by excellent blood circulation and generally minimal uptake in the lungs, liver, and spleen. Both CRX EVs and MSC EVs showed similar homing to injured tissues following lethal radiation; however, functionally, stimulating MSCs with a TLR-4 agonist enhanced the efficacy of the derived EVs compared to unstimulated MSC EVs. The radioprotective effects observed in two animal models of lethal systemic radiation toxicity suggest the potential of CRX Ev to reduce systemic radiotoxicity from radiopharmaceutical therapy.