Abstract
2356
Introduction: Worldwide, the incidents of melanoma have steadily increased over the last 50 years. In the US, the annual newly diagnosed cases of invasive melanoma increased by 44% in the past decade. Despite recent advances in treatments, the prognosis for late-stage melanoma remains particularly poor, with an average five-year survival rate of 22.5% for instances of metastatic disease. Since traditional chemotherapy for metastatic melanomas shows poor efficacy, targeted radionuclide therapy (TRT) with emerging powerful radionuclides offers an interesting alternative for treating advanced disease.
The melanocortin 1 receptor (MC1R) is specifically expressed in most melanomas, while normal healthy tissues show no significant expression, making it an interesting target for TRT development. Synthetic α-melanocyte-stimulating hormone (αMSH) analogues are peptides with high affinities for MC1R, and have been labeled with 18F, 68Ga, 177Lu, 212Pb, and 225Ac and have demonstrated efficacy for imaging or therapy.
Terbium (Tb) is one of the few elements which possesses isotopes suitable for both imaging and therapy, enabling theranostics with chemically identical radiopharmaceuticals. 161Tb (t1/2 6.89 d) is a promising therapeutic isotope that emits beta-particles along with Auger and conversion electrons. 155Tb (t1/2 5.32 d) is a SPECT imaging isotope with Auger and conversion electrons. In this report, we demonstrate for the first time the radiochemistry and initial preclinical studies of 161Tb and 155Tb labeled crown-αMSH, a MC1R targeting peptide conjugated with a non-conventional chelator.
Methods: Crown-αMSH, a chelator conjugated peptide targeting MC1R, was synthesized. The labeling conditions with 155Tb were investigated. Stabilities of the labelled peptide in buffer and human serum were studied. The in vivo uptake in mice bearing B16F10 tumors was examined for both 161Tb-crown-αMSH and 155Tb-crown-αMSH. SPECT imaging for 155Tb-crown-aMSH was recorded.
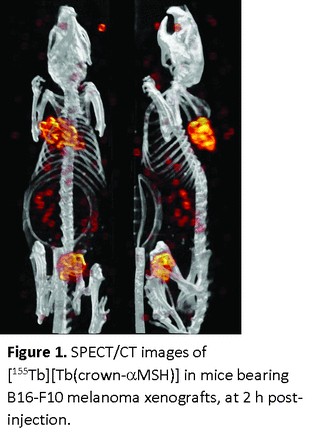
Results: Crown-αMSH can be labeled efficiently with both 161Tb and 155Tb (≥88% at 10-5 M) at ambient temperature under mild conditions. 155Tb-crown-αMSH exhibits impressive stability in solution, maintaining > 97% radiochemical integrity when challenged in human serum or aqueous buffer over 7 days. 161Tb and 155Tb-crown-αMSH showed very similar biodistribution profiles at 2 hours post injection in B16F10 tumor bearing mice. For both radiopharmaceuticals, we observed effective tumor accumulation, good image contrast and low uptake in other tissues and organs (Figure 1).
Conclusions: 161Tb/155Tb labeled crown-αMSH is a promising TRT agent for melanoma imaging and therapy and warrants further preclinical investigations.