Abstract
1701
Objectives: Recently great interest has been gained regarding fibroblast activation protein (FAP) as an excellent target for theranostics. Several FAP inhibitor molecules such as 68Ga-labelled FAPI-02 [1], 04 [1], 46 [2] and DOTA.SA.FAPi [3,4] have been introduced and are highly promising molecular targets from the imaging point of view. FAP inhibitors introduced via bifunctional DOTA [5] and DOTAGA chelators offer the possibility to complex 177Lu due to an additional coordination site and are suitable for theranostic applications owing to the increased tumor accumulation and prolonged tumor retention time. However, for therapeutic applications, very little has been accomplished in the therapeutic aspects mainly due to the residence times of the compounds. The aim this pilot study is to report the first clinical experience with 177Lu-DOTAGA.(SA.FAPi)2 therapy as a salvage treatment option in advanced stage radioiodine-refractory differentiated thyroid cancer (RR-DTC) patients.
Methods: Six patients (median age:61.5, range:46-67 years, males-2, females-4) with metastatic RR-DTC were prospectively recruited after disease progression was observed on standard treatments, including tyrosine kinase inhibitors such as Sorafenib and Lenvatinib. All patients had received >22.2GBq of radioiodine, progressed on both sorafenib followed by Lenvatinib. These patients underwent screening with 68Ga-DOTAGA(SA.FAPi)2 and 18F-FDG-PET/CT scans to confirm high CAF expression. Once deemed eligible for treatment, they received intravenous 177Lu-DOTAGA.(SA.FAPi)2 at eight-weekly intervals. The primary endpoint was assessment of biochemical response by thyroglobulin assessment. Secondary endpoints included response assessment according to the visual analog score (VAS), ECOG status, and safety assessment by the National Cancer Institute’s Common Toxicity Criteria V5.0.
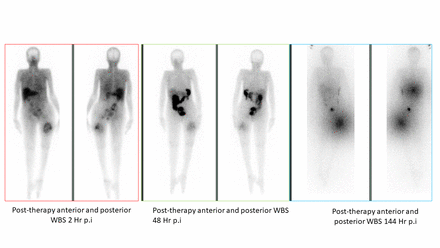
Results: Between October 2020 to January 2021, a total of 7 cycles were delivered to these patients. Only one patient received 2 cycles (Figure1). In a median follow-up duration of 2 months (range: 1-3 months), the mean administered radioactivity was 5.5 GBq per cycle (range 4.3 to 11 GBq). 66% (4/6) patients had bone metastases an lung metastases and only one patient had liver metastases. All patients experienced a remarkable decline in the median thyroglobulin levels from the baseline (baseline: 7839 ng/mL, 8 weeks post 1st cycle therapy: 6307 ng/mL, P-0.031). All four patients with bone pain experienced improvement in pain during treatment. The median time for the onset of pain relief was 5 days (range: 3-7 days) and all patients experienced sustained pain relief even at 8 weeks post-treatment. Improvement in the VASmax, and ECOG scores from the baseline was an indication of clinical benefit. At a median of 8 weeks after the first cycle of 177Lu-DOTAGA.(SA.FAPi)2 therapy, there was a significant improvement in the VASmax scores [pre-therapy: 8.6±1 vs. 8 weeks after 1st therapy: 5.5±0.8 (P-0.006)], and ECOG performance status [3.5 ± 0.8 vs. 2.5±0.54 (P-0.001)]. None of the patients experienced grade III/IV hematological, kidney or hepatotoxicity from [177Lu]Lu-DOTAGA(SA.FAPi)2 therapy. CONCLUSION: Biochemical response with thyroglobulin decrease at 8 weeks after 177Lu-DOTAGA.(SA.FAPi)2 initiation is an early predictor of better clinical outcome. Preliminary results suggest 177Lu-DOTAGA.(SA.FAPi)2 effective in pain palliation. 177Lu-DOTAGA.(SA.FAPi)2 therapy adds a new dimension to the treatment of RR-DTC patients who have exhausted all standard line treatment options. Figure 1: Biodistribution of 177Lu-DOTAGA.(SA.FAPi)2 at serial time-points in a RR-DTC patient (65 year old female) with skeletal metastases (orange arrow). There is rapid accumulation of radiotracer seen in the 2 hour image with retention up to 144 hours post-injection.