Abstract
1526
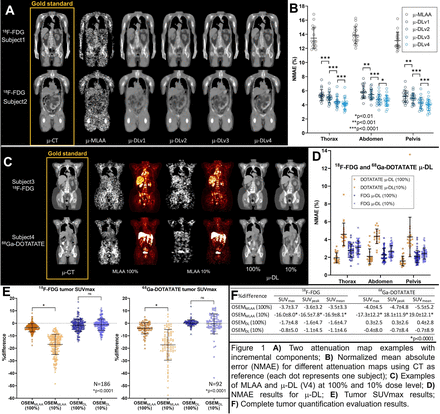
Objectives: In PET/CT scans, in addition to PET alone, radiation from CT is significant. While low-dose (LD) CT protocols may introduce artifacts into PET through attenuation correction, much effort has been made to leverage deep learning (DL) neural networks to synthesize CT or attenuation map (μ-DL) directly from PET data1. In the past, we have devised a novel DL framework2, which used MLAA3 emission and transmission as network input, to generate μ-DL, which was tested on clinical full-dose 18F-FDG datasets3. In this study, we demonstrated the benefits brought by each technical innovation2 to μ-DL, applied the optimized framework2 to synthetic LD (through listmode down-sampling) 18F-FDG and 68Ga-DOTATATE studies and evaluate PET tumor quantification.
Methods: One hundred full-body (skull-toe) FDG datasets (80/20 for training/testing, 370MBq injection) were used to demonstrate technical innovations. Another 87 FDG (40/47) and 62 DOTATATE (40/22) neck-thigh datasets with apparent tumors were used for LD studies. All data were acquired from a Siemens Biograph mCT scanner. CT was acquired before each PET study. A modified 3D U-Net implementation2 was used. Transmission and emission maps from MLAA3 were used as the network input. Using CT attenuation map (μ-CT) as training label, respective network was trained for each imaging paradigm at each dose level to generate μ-DL. Siemens e7 OSEM reconstruction was used to reconstruct PET using μ-DL and transmission map from MLAA. Tumor region of interests (ROIs) (N=186 for FDG and 92 for DOTATATE) were segmented by radiologists using an in-house image analysis tool (“Metavol2”). Network innovation was demonstrated by incrementally adding the following components to a reference network (V1, similar to4): more network parameters (V2, same number as in2), additional gradient loss, line-integral loss and novel normalization (V3, same as in2), and larger (64x64x32 voxels) training patch (V4). V4 was used for LD studies. μ-DL at 100% and 10% dose levels were compared to μ-CT; PET reconstructions (OSEMDL) of 100% (370MBq for FDG, 2.0MBq/kg for DOTATATE) and 10% levels were compared to CT-based reconstructions (OSEMCT) at the same dose levels, respectively. Normalized mean absolute error (NMAE) was used to evaluate attenuation map. Tumor SUVmax, SUVpeak (top 30% voxels in uptake) and SUVmean were reported.
Results: Networks with added components yielded superior μ-DL quality, both qualitatively (fig 1A) and quantitatively, i.e., NMAE for V1-V4: 5.5±0.7%, 5.2±0.6%, 4.5±0.6%, 4.2±0.7% (fig 1B). For the LD FDG study, μ-DL at 10% dose level showed similar quality (mild details loss, fig 1C) as compared to 100% level (NMAE 2.7±0.5% vs. 2.4±0.5%, fig 1D). OSEMDL at 10% level showed excellent performance in FDG tumor quantification (fig 1E, F), i.e., error in SUVmax, SUVpeak, SUVmean: -0.8±5.0%, -1.1±4.5%, -1.1±4.6%. For DOTATATE, larger μ-DL error was shown at 10% (NMAE 4.4±1.0%) as compared to 100% level (1.9±0.5%). OSEMDL at 10% dose level showed larger variability (fig 1F) in DOTATATE tumor quantification (SUVmax, SUVpeak, SUVmean: -0.4±8.0%, -0.7±8.4%, -0.7±8.9%) as compared to 100% level (0.3±2.5%, 0.3±2.6%, 0.4±2.8%). OSEM reconstruction using transmission map from MLAA was shown to be sensitive towards low-dose condition for both tracers (fig 1F). Conclusion: In this study, we demonstrated quality improvements in deep learning-based attenuation map (μ-DL) due to neural network innovations. μ-DL at 10% clinical dose level yielded excellent PET quantification with minimal error in 18F-FDG tumor SUV measurement. Larger variability in tumor quantification was found for 68Ga-DOTATATE using μ-DL at 10% level as compared to full dose. Reference: [1] Lee. 2020, https://doi.org/10.1109/TRPMS.2020.3009269 [2] Shi et al. 2019, https://doi.org/10.1007/978-3-030-32251-9_79 [3] Rezaei et al. 2012, https://doi.org/10.1109/TMI.2012.2212719 [4] Hwang et al. 2019, https://doi.org/10.2967/jnumed.118.219493