Abstract
We describe a unique case of a patient presenting with unilateral mild paresis, slowing of the upper limb, and parkinsonism, who underwent a full imaging work-up including MRI, 123I-FP-CIT PET, 18F-FE-PE2I PET, and 18F-FDG PET. This case demonstrates that imaging may aid substantially in the diagnostic work-up of complex neurologic disorders.
PART 1
Case Report
We report the case of a 60-y-old man who initially presented after 6 mo of subacute and slowly progressive symptoms including muscle stiffness, decreased fine motor skills, slowing of movements, and mild muscle weakness in his right hand. The patient was right-handed and experienced writing difficulties—without cramps or dystonia—that affected legibility. He had no complaints of postural symptoms associated with orthostatic hypotension or other autonomic failure. No sleep disturbance, periodic limb movement during sleep, or restless legs syndrome was mentioned.
The patient had a history of a myocardial infarction, diabetes mellitus, and hypercholesterolemia; the latter two were relatively well controlled with medication. His family history was negative for neurodegenerative diseases.
The neurologic examination showed slowness in whole-hand grasping and on tapping tasks, as well as mild paresis of the dorsal interosseous muscles of the right hand. Furthermore, we found an asymmetric mild hypertonia in the lower right limb. A unilateral positive Hoffman sign (right) was noted, but we found no Babinski sign and tendon reflexes were symmetrically brisk. When the patient walked, we observed a reduced arm swing on the right side without postural imbalance or gait disorder. There was no evidence of myoclonus. All sensory modalities were normal. Eye movements, both saccades and vertical gaze, were normal. Furthermore, detailed language testing and a behavioral assessment showed no abnormalities. Obtaining a diagnosis remained challenging, as it was difficult to distinguish between parkinsonism and a motor neuron disorder.
Hematologic and serum biochemistry tests had normal findings. The electromyography results were strictly normal.
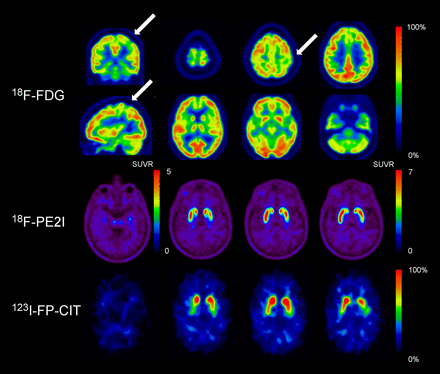
MRI of the brain demonstrated mild white matter lesions normal for his age, without any hyperintensity in the corticospinal tracts. The cervical MR images showed no evidence of myelopathy or radiculopathy. 18F-FDG brain PET showed asymmetric mild hypometabolism in the left sensorimotor cortex compatible with the right arm symptoms, in favor of a motor neuron disease. To exclude neurodegenerative parkinsonism 123I-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane (123I-FP-CIT) SPECT was performed 9 mo later and showed normal presynaptic dopaminergic transmission (Fig. 1).
(Top) Orthogonal 18F-FDG PET slices showing mild hypometabolism (arrows) in left sensorimotor cortex corresponding to right arm location of cortical homunculus. (Middle and bottom) Transverse slices of dopamine transporter imaging. Both classic 123I-FP-CIT SPECT and high-resolution, selective 18F-PE2I PET showed normal presynaptic dopaminergic uptake. Images are in radiologic orientation. SUVR = SUV ratio.
Notably, there was no response to dopaminergic treatment with levodopa and benserazide.
Discussion
The differential diagnosis was broad. After excluding structural, metabolic, inflammatory, and infectious disorders, we were leaning toward diagnosis of a motor neuron disorder. Finally, we found no mutations in the SOD1, TARDBP, FUS, or C9orf72 genes. Whole-exome sequencing was done but showed no mutations related to neurodegenerative disorders.
Besides a slow progression of isolated paresis of the right hand, the patient’s condition remained unchanged during a 1-y follow-up period. Repetition of the electromyography showed no signs of lower motor neuron degeneration. We requested a more sensitive 18F-(E)-N-(3-iodoprop-2-enyl)-2β-carbofluoroethoxy-3β-(4′-methyl-phenyl) nortropane (18F-FE-PE2I) PET scan to exclude subtle presynaptic dopaminergic transmission deficits. This imaging showed no abnormalities in the striatum or in the substantia nigra.
PART 2
Final Diagnosis
Together, the clinical picture and ancillary tests were compatible with an asymmetric form of pure upper motor neuron disorder. The imaging allowed diagnosis of primary lateral sclerosis (PLS) by showing on the one hand a hypometabolism of the sensory-motor cortex contralateral to the clinical involvement and on the other hand a presynaptic integrity of the dopaminergic pathways.
Conclusion
PLS is a rare neurodegenerative disorder characterized by a slowly progressive upper motor neuron syndrome. Compared with classic variants of amyotrophic lateral sclerosis, PLS has a significantly slower rate of disease progression and longer survival. Diagnosis of PLS is challenging and is based on excluding structural disorders (e.g., cervical spondylotic myelopathy, Arnold–Chiari malformation, spinal arteriovenous fistula, and tumor), hereditary spastic paraparesis, leukodystrophies, metabolic and toxic disorders (e.g., vitamin E deficiency, cerebrotendinous xanthomatosis, and hexosaminidase deficiency), neurodegenerative disorders (e.g., Parkinson disease, multiple systems atrophy, and corticobasal syndrome), inflammatory disorders (e.g., primary progressive multiple sclerosis), and infections (e.g., neurosyphilis, tropical spastic paraparesis, neuroborreliosis, spinal sarcoidosis, and AIDS). Thalamic, hippocampal, and basal ganglia atrophy, as well as subcortical gray matter degeneration, have been demonstrated in imaging studies of PLS (1). Postmortem studies have reported atrophy of the thalamus and striatum, as well as TDP-43 inclusions in the striatum, amygdala, hippocampus, and basal ganglia.
In this case, molecular imaging had a substantial added value to aid in the differential diagnosis between neurodegenerative parkinsonian syndromes and amyotrophic lateral sclerosis. Neurodegenerative parkinsonian syndromes are characterized by a presynaptic dopaminergic deficit. 123I-FP-CIT SPECT is widely used in clinical practice to visualize dopaminergic system integrity at the level of the presynaptic terminals in the striatum (2). 18F-FE-PE2I PET is a novel highly selective and high-resolution DAT imaging technique that allows visualization and quantification of both the striatum and the substantia nigra (3). As both techniques, when repeated after 12 mo of follow-up, had normal results, a presynaptic dopaminergic deficit could be excluded. Although dopaminergic deficits have been described in amyotrophic lateral sclerosis patients, a recent report on PLS patients with atypical signs of parkinsonism did not find any dopaminergic deficits (4), in line with our results.
Moreover, 18F-FDG PET also showed typical signs of a motor neuron disease, as a mild hypometabolism in the left sensorimotor cortex was found corresponding to the right arm location of the homunculus (5,6). Therefore, advanced brain imaging guided the diagnosis.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 20, 2021.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 22, 2021.
- Accepted for publication April 13, 2021.