Abstract
137
Objectives: [11C]CO2 fixation strategies have recently been applied for the synthesis of 11C-carbonylated radiopharmaceuticals. Whereas 11C-carbamates have been translated for human PET studies, the syntheses of 11C-carboxylic acids via [11C]CO2 fixation has proven to be challenging. A novel copper-mediated carboxylation strategy of aryl- and heteroaryl-stannanes is described as a mild (i.e. 1 atm) carboxylation method using CO2 and is transferable for carbon-11 labeled aromatic and heteroaromatic carboxylic acids using [11C]CO2. The methodology was applied to the radiosynthesis of a retinoid X receptor (RXR) agonist, [11C]bexarotene.
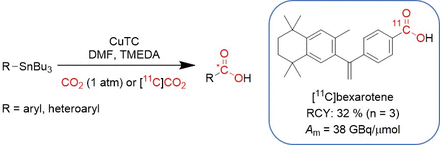
Methods: Feasibility of the carboxylation reaction was first tested by reactions in a sealed vessel under an atmosphere of CO2. Translation of the method for [11C]CO2 involved varying the reaction temperature, solvent, Cu(I) source, and base, using PhSnBu3 as precursor. The preparation of [11C]benzoic acid was optimized as follows: temperature: 100 °C; time: 5 minutes; solvent: DMF (600 μL); [PhSnBu3] = 0.1 M; [CuTC] = 9.4 mM; and [TMEDA] = 1.3 M. Substrate scope was evaluated using tributyltin compounds substituted by para-substituted benzene derivatives (pMe, pMeO, pCl, and pCF3), followed by compounds substituted with 2-furan, 2-thienyl, and N-heteroarenes (3-pyridyl, 2-pyrazyl, 2-pyrimidyl and 5-pyrimidyl). The new carboxylation method was applied in the fully automated radiosynthesis of [11C]bexarotene using the TracerMaker (Scansys Laboratorieteknik, Denmark) synthesis module.
Results: [11C]Benzoic acid was isolated in a decay-corrected RCY of 45% relative to starting [11C]CO2, with molar activity Am = 9.3 GBq/µmol (250 mCi/µmol), and radiochemical purity >99%, in 29 min. Decay-corrected radiochemical yields (RCYs) of ca. 30-70% were obtained with arylstannanes (PhSnBu3 and its corresponding pMe, pMeO and pCF3 substituted derivatives). Formation of 11C-carboxylic acids bearing 3-pyridyl, and 2-pyrazyl groups were successfully generated with RCYs between 32-34%, while yields for the transfer of electron-rich heteroarenes 2-furyl and 2-thienyl were generally lower (<3%). [11C]Bexarotene was synthesized and formulated in a decay-corrected RCY of 32 ± 5% (n = 3), relative to starting [11C]CO2, with molar activity Am = 38 ± 23 GBq/μmol (1.0 ± 0.6 Ci/μmol) and radiochemical purities >95% in 41 ± 4 minutes from EOB. Isolated formulations (10% ethanol/90% saline v/v) from scaled up reactions contained up to 5.74 GBq (155 mCi) of activity and high molar activity (Am = 63 GBq/μmol; 1.7 Ci/μmol).
Conclusions: We have developed a novel copper-mediated carboxylation methodology for the preparation of (hetero)aryl carboxylic acids from organostannanes using a stoichiometric quantity of CO2 under atmospheric pressure, as well as sub-stoichiometric quantities of [11C]CO2. The successful radiosynthesis, purification, and formulation of [11C]bexarotene, using a fully automated commercial synthesis module, demonstrates feasibility of 11C-carboxylation reactions in radiopharmaceutical production.