Abstract
608
Objectives: Co-administration of additional therapies, such as external beam radiation, with checkpoint immunotherapy may result in modulation of checkpoint molecule expression. This process is highly dynamic and difficult to capture with traditional biopsy-based analysis; therefore, molecular imaging, particularly positron emission tomography, may enable real-time insight into these expression levels. The human/mouse cross-reactive anti-PD-L1 antibody atezolizumab was utilized to visualize tumor PD-L1 expression changes following various external beam radiotherapy interventions.
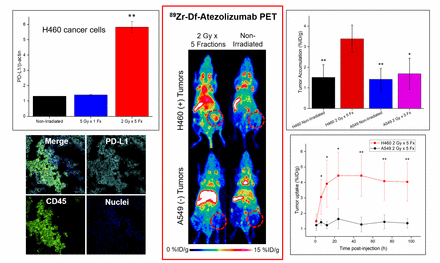
Methods: A humanized anti-PD-L1 antibody, atezolizumab, was radiolabeled with 89Zr (half-life: 78.4 h) for PET imaging following external beam radiation to PD-L1-expressing (H460) or PD-L1-negative (A549) lung cancer cells or tumor xenografts in mice. Using a biological irradiator, three schedules were utilized: 5 Gy in a single dose; five daily fractions of 2 Gy; or no irradiation. One day after completion of the respective radiotherapy regimens, mice were injected with 89Zr-Df-atezolizumab and serial PET imaging was performed out to 120 h post-injection. Biodistribution and histology studies were conducted to verify the in vivo findings, and tracer accumulation differences between irradiated groups and non-irradiated, as well as positive and negative tumors, were analyzed for statistical significance.
Results: In vitro studies verified increased expression of PD-L1 by H460 cells following radiotherapy, particularly with the fractionated regimen, while PD-L1 expression could not be induced on A549 cells with the employed techniques. Imaging with the PD-L1-targeted tracer revealed high signals in the spleen and lymph nodes of all imaged mice (~10-20 %ID/g). Tumor accumulation was highest in the H460-bearing mice receiving 5 fractions of 2 Gy at 24 h post-injection at 4.44 ± 1.52 %ID/g, compared to that observed in the negative A549 fractionated regimen mice at 1.64 ± 0.65 %ID/g (p < 0.02, n=4-6). Both positive and negative non-irradiated tumors revealed similar accumulation of 89Zr-Df-atezolizumab at the end of the study, with uptakes of 1.51 ± 0.61 %ID/g in H460 tumors, and 1.42 ± 0.95 %ID/g in A549. Ex vivo analysis of tissues demonstrated significant PD-L1 expression in lymphoid organs, correlating with the high tracer accumulation in these tissues. In H460 tumors, PD-L1 expression by both tumor and myeloid cells was observed, and PD-L1 staining was minimal in A549 tissues.
Conclusions: While the absolute level of 89Zr-Df-atezolizumab accumulation in tumor tissues was low due to the presence of notable sink tissues, significant differences were noted between treated and untreated tissues. PD-L1 imaging may therefore play a unique role for monitoring of treatment efficacy by comparing the dynamics of immune checkpoint expression before, during, and after therapeutic intervention.