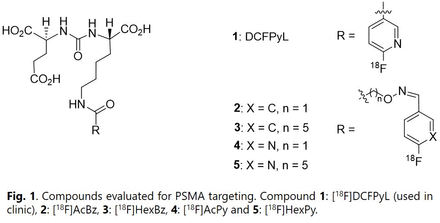
Abstract
343
Objectives: PSMA is highly expressed on prostate cancers and is an attractive biomarker for targeted imaging and therapy using small molecules containing the PSMA inhibitor lysine-urea-glutamate binding motif. A PET imaging agent, [18F]DCFPyL (1), clinically used for targeting PSMA demonstrates high affinity and specificity for PSMA resulting in quality image resolution. However, [18F]DCFPyL accumulates and is retained in the kidneys, which makes detection of PSMA lesions near the kidneys challenging. Further, the kidneys become the dose limiting organ for radiotherapy. Recently, a [18F]DCFPyL analogue, [18F]AcBz (2), demonstrated improved PSMA targeting but slower kidney clearance compared to 1, thus has inspired the development of other [18F]DCFPyL analogues [1]. Herein, novel oxime-linked fluorine-18 radiolabeled analogues ([18F]HexBz (3), [18F]AcPy (4), and [18F]HexPy (5)) were developed and compared in vitro and in vivo using PC3-PSMA+ PCaX tumor mouse models to potentially improve kidney clearance and tumor targeting.
Methods: Fluorine-18 radiolabeled DCFPyL (1), 4-[18F]fluorobenzaldehyde and 6-[18F]fluoronicotinaldehyde were prepared following published methods [2, 3, 4]. Oxime formation of aminooxy functionalized PSMA-inhibitor lysine-urea-glutamate scaffold with fluorine-18 labeled aldehydes produced radiotracers 2-5 (Fig. 1), which were purified by HPLC and had radiochemical yields between 25-40% (n > 10, uncorrected) within 50-60 min. In vitro saturation and competition binding studies of 1-5 were performed using PC3-PSMA+ cells to evaluate affinity (Kd; Ki). In vivo biodistribution studies of 1-5 were performed using PC3-PSMA+ PCaX mice at 1 h post injection (p.i.) for positive (radiotracer only) or blocking (radiotracer with 1000x concentration of non-radioactive DCFPyL) groups, from which % injected doses per gram of tissue (% ID/g) were determined. PET/CT imaging studies were collected at 1 h p.i. using the same PCaX model. Thin-layer-chromatography (TLC) was performed with serum samples 1 h p.i. to evaluate the percent intact radiotracer (% parent).
Results: Kd and Ki values of 1 (0.23-0.32 nM) were similar within 2 fold of 2-4 (0.10-0.31 nM) while 5 (0.7-0.9 nM) was 3 fold higher than 1. Biodistribution of 1-5 at 1 h p.i. demonstrated tumor uptakes ranging from 15-28% ID/g which were 57-91% blocked (1.5-12% ID/g). Tissue-to-blood (T:B) and tissue-to-muscle (T:M) ratios revealed similar tumor blocking ranging from 59-83% for 1, 3, and 5, however less blocking was observed for 2 (28-42%) and 4 (16-26%). Kidney uptake ranged from 144-153% ID/g for all compounds except 3, which was reduced by ~40% (~90% ID/g). Increased liver uptake was observed for 2 (10-11% ID/g), 3 (8-9% ID/g) and 5 (18-20% ID/g) compared to 1 (2% ID/g) and 4 (3% ID/g) which were 3 to 10 fold lower. Serum TLC results (% parent) demonstrated in vivo stability was enhanced for 3 (73 ± 3%) and 5 (74 ± 2%) compared to 1 (43 ± 6%). Bone uptake of 2-4 were comparable to 1 whereas 5 was higher suggesting 1-4 have less in vivo defluorination. PET/CT imaging studies of compounds 1-5 confirmed the biodistribution results and tumors were easily visualized.
Conclusions: PSMA inhibitor compounds 1-5 demonstrated suitable in vitro affinity and in vivo tumor uptake for imaging. Compounds 2 and 4, with higher T:M and T:B ratios and similar PSMA retention compared to 1, may be more desirable imaging agents that are capable of detecting PSMA at lower concentrations. Compound 3 may serve as a better radiotherapeutic agent sparing dose to the kidneys without jeopardizing tumor uptake. References: [1] Bouvet V, Wuest M, Bailey JJ et al. Mol Imaging Biol 2017;19:923-932. [2] Basuli F, Zhang X, Woodroofe CC et al. J Label Compd Radiopharm 2017;60:168-175. [3] Basuli F, Zhang X, Jagoda EM et al. J Label Compd Radiopharm 2018;61:599-605. [4] Bouvet V, Wuest M, Jans H et al. EJNMMI Research 2016;6:40.