Abstract
275
Objectives: While cancer immunotherapy has revolutionized the treatment of certain cancers, overall response rates remain low. In part, this is due to the complex interplay between the immune system and the tumor microenvironment. Although techniques such as flow cytometry and cytokine analysis are highly informative, their destructive nature limits the longitudinal information provided. Molecular imaging, conversely, can provide a non-invasive and quantitative examination of specific processes of interest. Previously, we developed a granzyme B PET imaging agent and demonstrated specific detection that was predictive of response to immunotherapy. Based on these findings, we utilized PET imaging in combination with flow cytometry and cytokine analysis in order to better understand the conditions and potential factors that lead to high granzyme B release.
Methods: Mice bearing CT26 or MC38 syngeneic tumors underwent granzyme B PET/CT imaging at 6 or 12 days post-initiation of anti-PD-1 plus anti-CTLA-4 combination therapy, and tumor and blood pool was quantified by drawing a three-dimensional region of interest using CT guidance. Tumor-specific accumulation was then calculated using the tumor to blood ratio (TBR). After the completion of PET imaging, tumors were excised, and a single-cell suspension generated. The supernatant of this suspension was saved for cytokine analysis using a 36-Plex Mouse Cytokine analysis kit. Cells were stained with antibodies to differentiate T cell subtypes and activation states and flow cytometry performed. As higher granzyme B PET TBR is consistent with subsequent response to immunotherapy, the TBRs were plotted against individual flow cytometry and cytokine results to explore correlations between immune cell types and cytokines and granzyme B release.
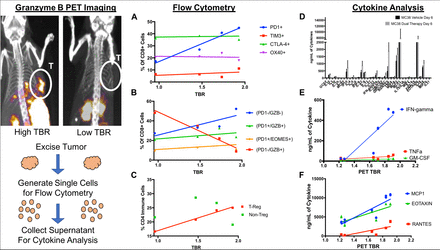
Results: PET imaging resulted in tumors with granzyme B PET TBRs ranging from 0.95 to 2.41, which was consistent with previous measurements following combination immunotherapy. When individual tumor granzyme B TBR was compared to the corresponding immune cell populations and cytokine expression, several correlations were observed. Among immune cell populations, there was a positive linear correlation between PD-1 expression and granzyme B PET signal (Figure A) but a negative correlation between granzyme B signal and PD-1-negative, granzyme B-positive CD8 T cells (Figure B). This indicates that actively tumor-killing T cells express PD-1 but become devoid of intracellular granzyme B, most likely because they have released a majority of their granules. Regulatory T cells were also positively correlated with granzyme B PET signal (Figure C), which may be a function of a negative feedback loop signaling recruiting following cytotoxic T cell activation. In addition to flow cytometry, cytokine expression was also quantified. Granzyme B PET signal was compared to concentration of 36 TH1, TH2, or TH17 cytokine (Figure D). Some expected cytokines positively correlated with granzyme B PET TBR, including IFN-gamma, TNF-alpha and GM-CSF (Figure E), but other unexpected cytokines with roles in macrophage, helper T cell and eosinophil chemotaxis were also positively correlated (Figure F).
Conclusions: Granzyme B PET imaging combined with ex vivo analysis provides a unique insight into factors that drive response to immunotherapy. In our analyses, we observed correlations between granzyme B and CD8 T cell activation/exhaustion and regulatory T cell presence. Additionally, multiple cytokines were positively correlated with granzyme B PET signal including expected cytokines like IFN-gamma and TNF-alpha, and also new cytokines involved in the trafficking of cells that are not traditionally associated with the anti-tumor immune response. These finding form the basis for new potential avenues of therapeutic intervention and suggest further investigation into the role of non-T cell immune cells in the anti-tumor immune response.