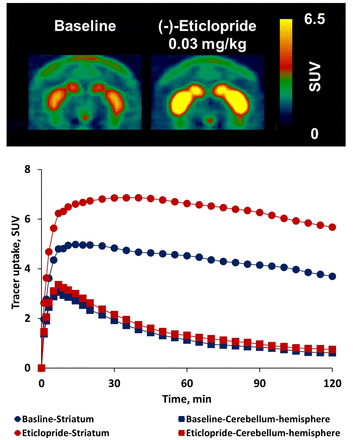
Abstract
340
Objectives: Cholinergic deficits are associated with cognitive dysfunction and dementia in people with disorders such as Alzheimer disease, Parkinson disease, and progressive supranuclear palsy (PSP). Quantification of cholinergic neurons could provide insight into the pathophysiology or treatment of these conditions. PET measures of vesicular acetylcholine transporter (VAChT) may permit in vivo measurement of cholinergic neurons. [18F]VAT, a radioligand with favorable kinetics for measuring VAChT [1] potentially could be affected by dopamine D2-like drugs that are commonly used in these human disorders. Herein, we report our initial investigations to determine the impact of D2 antagonist on VAChT expression in the brain of nonhuman primates using PET with [18F]VAT. Methods [18F]VAT was synthesized as previously reported [1]. Three male adult cynomolgus macaques underwent PET scans with a Focus-220 microPET. After intravenous (iv) injection of 8-10 mCi of [18F]VAT, either baseline scans or one blocking study with (-)-vesamicol (0.125 mg/kg, iv, 5 min prior to tracer injection) were performed with arterial blood sampling. A second series of studies included pretreatment 5 min prior to tracer injection with escalating doses of a D2 antagonist (-)-eticlopride ranging from 0.01 to 0.03 mg/kg. The interval between two consecutive scans on the same subject was at least 4 weeks. Imaging data process were performed to generate parameters including standardized uptake value (SUV), volume of distribution (VT), non-displaceable volume of distribution (VND) and non-displaceable binding potential (BPND). Results Lassen plot was used to validate cerebellar hemispheres as the reference region for kinetic analysis in the (-)-eticlopride pretreatment studies. The VT value of cerebellar hemispheres had negligible change after partial blockade with 0.125 mg/kg (-)-vesamicol. The VND value was 1.27 mL/cm3 and was undistinguishable from baseline VT value of cerebellar hemispheres, indicating that cerebellar hemispheres could be a suitable reference region for [18F]VAT. Tissue-activity curves showed that pretreatment with high dose (-)-eticlopride (0.03 mg/kg) increased [18F]VAT uptake in striatum, but not in cerebellar hemispheres. Low dose (-)-eticlopride (0.01 mg/kg) had no significant impact on [18F]VAT brain uptake. Striatal BPND values from baseline, 0.01 and 0.03 mg/kg (-)-eticlopride pretreatment studies were 3.82, 3.49, and 5.12 using simplified reference tissue model (SRTM), and 3.50, 3.40, and 5.05 using Logan Reference model (LoganREF). Conclusion D2 antagonism by (-)-eticlopride at high dose (0.03 mg/kg) dramatically increased striatal uptake of [18F]VAT in NHPs, suggesting PET with [18F]VAT provides a useful tool for investigation of dopaminergic modulation on VAChT expression in the brain of living animals. The elevation of striatal [18F]VAT uptake by 0.03 mg/kg (-)-eticlopride might be attributed to the disinhibition of cholinergic transmission by D2 antagonism. Ongoing studies are exploring the impact of D2 agonism on VAChT expression in the NHP brain. These results may help guide future investigation of the effects of dopaminergic drugs on cholinergic function in humans. Research Support NS075527, NS103988. Reference 1. Tu, Z., et al. Bioorg. Med. Chem. 2015, 23, 4699-709. Figure 1. Representative PET images (top panel) and time-activity curves (lower panel) showing striatal tracer uptake of [18F]VAT was upregulated dramatically by the pretreatment with 0.03 mg/kg (-)-eticlopride.