Abstract
1602
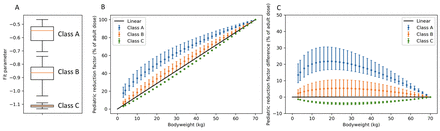
Objectives: n comparison with adult protocols, radiopharmaceutical activities administered in pediatric studies require extra attention due to the increased risk of long-term radiation effects. Moreover, in order to obtain the desired diagnostic image quality, less activity is required. Clinical practice guidelines typically focus on the administered activities for adults. However, the administered activity for pediatric patients can and should be lower than the activity administered in adults, which can be done using a pediatric reduction factor. To calculate this factor several methods can be used, including scaling with a factor proportional to the body surface area or body weight, as recommended by the North American Consensus Guidelines (1). Other methods aim to keep the effective dose or the count rate constant. The pediatric dosage card published by the European Association of Nuclear Medicine (EANM) scales the adult activities with a reduction factor that aims to keep the effective dose constant (2,3). The EANM dosage card tabulates reduction factors for different body weights between 3 and 70 kg. These reduction factors aim to keep the effective dose equal, regardless of the body weight of the patient and were obtained in the following way. The effective dose was determined for 95 different tracers for children of 1, 5, 10 and 15 years by consulting the International Commission on Radiological Protection (ICRP) publications. Subsequently, normalization factors that keep the effective dose equal were determined by dividing the pediatric effective dose by the adult effective dose for each tracer. The normalization factors that depend on the body weight were fitted with an exponential function (factor =(Weight/70)-a). This resulted in a fit parameter for each individual tracer. The fit parameters were used to group the tracers (class A, B and C) and mean fit parameters were determined for each class. Finally, the pediatric reduction factors were calculated for each class based on the mean fit parameter, resulting in the three tables of pediatric reduction factors for different body weights. The purpose of this current study was to show that for many isotopes the pediatric reduction factors used to keep the effective dose constant (i.e. the EANM dosage card) can be approximated with a straightforward linear function of body weight. This would simplify dosage calculation. Methods For this study, we plotted the pediatric reduction factors as a function of body weight. As a measure of the uncertainty, we added error bars to this graph that were calculated based on the 95% confidence interval of the fit coefficients of the individual tracers. Additionally, we calculated the difference between the pediatric reduction factor and the linear relation and plotted it as a function of body weight. Again, we added error bars based on the 95% confidence interval in the fit parameters. Results The results show a large variance of the scaling factors especially for classes A and B (Figure 1). Note that the actual uncertainty of the pediatric reduction factor is larger, as only the variance in fit parameters is taken into account but not whether an exponential function is a good approximation of the data. Additionally, we calculated the difference between the pediatric reduction factor and the linear relation and plotted it as a function of body weight. Classes B and C show a much smaller deviation from the linear function than class A. For example, if the adult activity is 100 MBq then the pediatric administered activity for a pediatric patient with a body weight of 30 kg would be 48 (43 - 53 95% CI) MBq according to the class B dosage card. Linear dosage would result in an activity of 43 MBq. Conclusion The pediatric reduction factors show large uncertainty. The pediatric reduction factors for classes B and C can be approximated using a linear function of body weight. This allows for a simplification of the pediatric dosage calculation, increasing the ease of use in clinical practice.