Abstract
1027
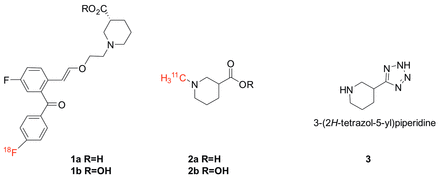
Objectives: PET imaging of the GABA transporter type 1 (GAT-1) is not yet possible due to a lack of appropriate radiotracers. Our previous efforts to synthesize GAT-1 selective PET radioligands were based on previously reported lipophilic nipecotic acid derivatives,1 and the presence of a free carboxylic acid group on these derivatives interfered with BBB permeability. Bioisosteres of carboxylic acids have long been used in CNS drug development. We will report the use of carboxylic acid bioisosteres to preserve selectivity while increasing BBB permeability in the development of a GAT-1 selective PET probe. Methods: Recent efforts have been focused on the radiolabeling and in vivo evaluation of both [18F]-labeled lipophilic nipecotic acid derivatives (1a,1b), and 11C-N-methylated nipecotic acid (2a) and its ethyl ester (2b). The lipophilic nipecotic acid derivatives were labeled as previously described and evaluated in both Sprague Dawley rats and non-human primates. Nipecotic acid ethyl ester was N-[11C]-methylated using [11C]-methyl triflate in DMF.2 The free N-[11C]methyl-nipecotic acid was formed by saponification of the ester with LiOH (0.5 mL, 100 °C, 5 min). Identities of radiotracers were confirmed by HPLC. The radiolabeled carboxylic acid and ethyl ester were evaluated by microPET imaging in Sprague Dawley rats. Concurrently, a tetrazole was found as the most promising in a preliminary in vitro study of commercially available bioisosteres. Following this lead, a 3-tetrazole piperidine (3) has been synthesized using published methods.3 Results: [18F]Fluorination and [11C]methylation reactions were performed manually or using an automated synthesis module. The lipophilic nipecotic acid derivative 1a was prepared in 13% radiochemical conversion (RCC) from the chloro precursor, while 2 was prepared in 24% (acid form) and 53% (ester form). MicroPET imaging showed that for each set, brain uptakes of free acids (1a,2a) were very low; whereas, measureable levels of the corresponding esters could be observed for both esters (1b,2b). Conclusions: The comparison of the simple nipecotic acid (2a) and ester (2b) with the larger lipophilic GAT-1 ligands (1a,1b) supports a conclusion that it is predominantly the free carboxylic acid function that limits BBB permeability. These results demonstrate the need for the development of carboxylic acid bioisosteres for use in these GAT-1 radioligands. Syntheses of analogs of 1a and 2a but with a tetrazole replacing the carboxylic acid (as in 3) are in progress. These syntheses will be discussed, as well as initial in vivo evaluation of new radio tracers. Acknowledgements: Research was supported by NIH grant R21NS086758. 1. G. Quandt, G. Hofner and K. T. Wanner, Bioorg Med Chem, 2013, 21, 3363-3378. 2. T. Nguyen, S.E. Snyder, and M.R. Kilbourn, Nucl Med Biol, 1998, 25, 761-768. 3. S. Harusawa, H. Yoneyama, Y. Usami and S. Komeda, Synthesis, 2013, 45, 1051-1059.