Abstract
1025
Objectives: PBT2 (5,7-dichloro-2-((dimethylamino)methyl)quinolin-8-ol) is a metal protein-attenuating compound (MPAC) that was developed for the treatment of Alzheimer’s disease(AD) and which has recently been repurposed for Huntington’s disease. Our objectives are to: (1) radiolabel PBT2 with carbon-11 in its native form at the N-methyl position; (2) determine the pharmacokinetic profile of [11C]PBT2 in rodents and nonhuman primates by PET imaging studies; and (3) evaluate [11C]PBT2 in post-mortem human AD tissue to assess specific binding. Methods: Carbon-11 labeled PBT2 was synthesized by reaction of [11C]CH3I with 5,7-dichloro-2-((dimethylamino)methyl)quinolin-8-ol via an automated method, employing a custom designed small-volume glass vial to fit a commercial radiofluorination module (GE Tracerlab FXF-N). This apparatus enables the use of a traditional automated radiofluorination apparatus for reactor-based carbon-11 labeling reactions. Starting with >37 GBq (>1 Ci) of [11C]CH3I, [11C]PBT2 was routinely synthesized PET imaging studies with [11C]PBT2 were performed in BALB/c mice and non-human primates, and preliminary in vitro autoradiography studies using human AD brain tissue was performed.
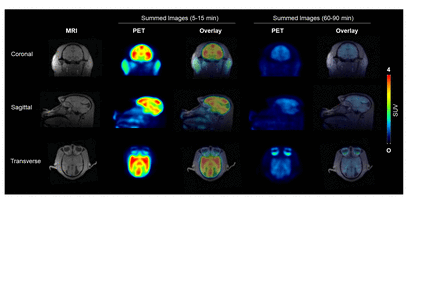
Results: The normethyl precursor 5,7-dichloro-2-((methylamino)methyl)quinolin-8-ol, for the synthesis of [11C]PBT2 was prepared in 4-steps from commercially available 5,7-dichloro-2-methylquinolin-8-ol. Radiolabeling with [11C]CH3I was achieved using a mixture of DMF and DMSO (2:1, v/v) for 7 min at 120 oC. [11C]PBT2 was isolated and formulated in 4.8 ± 0.5% (s.d., n = 6, non-decay corrected) radiochemical yield in ~50 min, with >99% radiochemical purity and molar radioactivityof 80-90 GBq/μmol (2200-2500 mCi/μmol). Dynamic small animal PET imaging data were acquired for 30 min and demonstrated that [11C]PBT2 rapidly permeates the blood-brain barrier (BBB) in mice following intravenous injection with peak SUV for the whole brain (SUVpeak) of 1.3 ± 0.3 (at 1.3 min post-injection, n = 2). The initial uptake was followed by fast washout of the radiotracer with SUVpeak/SUV30 min ratio of 6.3 and only negligible radioactivity remaining in the brain in the later phase (20-30 min post-injection). [11C]PBT2 displayed excellent brain penetration in the non-human primate brain following intravenous injection with an averaged SUVpeak in gray matter areas of 4.09 at 2.2-4.5 min post-injection, followed by a favorable and fast brain tissue clearance with convergence of all time-activity curves and uniform kinetics for all brain regions. In light of the promising in vivo PET imaging profiles observed, we carried out autoradiography studies and confirmed that [11C]PBT2 binds specifically to postmortem AD human brain cryo-sections (occipital cortex; Δ = -36.2 ± 2.7% under self-blocking conditions). Conclusions: The first radiosynthesis and preclinical PET neuroimaging evaluation of [11C]PBT2 revealed high uptake in all cortical and sub-cortical gray matter regions followed by rapid washout from normal brain tissues and specific binding was seen in AD human brain tissue in vitro. Future work will focus on detailed quantification, radiometabolite assessments and human PET evaluation. Combined with the safety, tolerability and favorable metabolic profiles of PBT2 established in human, we anticipate an accelerated path to [11C]PBT2 human PET studies.