Abstract
861
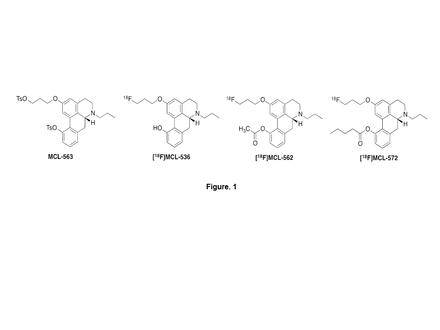
Objectives: Abnormality of dopamine D2 and D3 receptors is implicated in several neuropsychiatric disorders, including Parkinson’s disease and schizophrenia. Our earlier studies (Bioorg Med Chem. 2004, 12, 3553) showed that several non-radiolabeled 11-hydroxynoraporphines and their esters show high behavioral potency for D2 receptors in rodents, presumably due to their lipophilicity and prodrug nature. Here we report our more recent efforts to extend these results to the development and evaluation of [18F]-labeled D2 imaging agents based on the acetate ([18F]-MCL-562) and valerate ([18F]-MCL-572) esters of R-(-)-N-n-propyl-2-(3-fluoropropanoxy-11-hydroxynoraporphine) ([18F]-MCL-536) (J. Label. Compd. Radiopharm, 2014, 57, 725-729).
Methods: The desired radiotracers, [18F]-MCL-562 and [18F]-MCL-572 (Fig. 1), were synthesized from the same bis-tosylated precursor MCL-563, (R)-N-n-propyl-11-toluenesulfonyloxy-2-(3-toluenesulfonyloxypropanoxy)noraporphine. Following initial radiolabeling using standard radiofluorination procedures (i.e., K2.2.2, K2CO3, CH3CN, 110 °C), the compound was deprotected (3 M NaOH, 15 min, 70 °C), the resulting 3-[18F]fluoropropanoxy 11-OH compound ([18F]-MCL-536) was purified by semi-preparative HPLC, and the ester was generated using either acetic anhydride ([18F]-MCL-562) or valeric anhydride ([18F]-MCL-572) (room temperature, 5 min). The final product is isolated using a C18 cartridge and formulated in 10% ethanol/saline. LogD7.4 was measured by the shake-flask method. Non-invasive imaging was performed in rats using dedicated small-animal PET/CT system. In addition, the stability of the 11-hydroxyaporphine valerate ester was measured in human and rat serum.
Results: The radiotracers [18F]-MCL-562 and [18F]-MCL-572 were obtained with 5% decay-corrected radiochemical yield and >99% radiochemical purity (n=2) in a total synthesis time of 2 h. The specific activities were 5 GBq/μmol and 2.5 GBq/μmol, respectively. LogD7.4 was found to be 1.54+0.09. The PET images indicated both the tracers had no specific uptake in the rat brain. The in vitro stability study showed the valerate ester was <4% intact in rodent serum at 5 min and 54% intact in human serum at 5 min.
Conclusion: The radiotracers [18F]-MCL-562 and [18F]-MCL-572 were synthesized in modest radiochemical yield but high purity. Unfortunately, neither tracer showed high or specific uptake in the rat brain, presumably due to the rapid metabolism in the rat serum. However, based on in vitro human serum stability, the valerate ester of aporphine could be the promising candidate for more extensive evaluation and imaging studies are underway in non-human primates. Research Support: NIH-5R01HL108107, NIH-2R21MH103718, The Children’s Hospital Radiology Foundation and Branfman Family Foundation.