Abstract
405
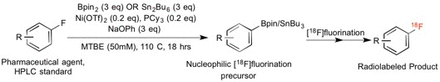
Objectives: Several published methods enable the conversion of fluoroarenes to their complementary boronic esters (Bpins), which are substrates for copper-catalyzed nucleophilic [18F]fluorination of electron-neutral and electron-rich arenas [1]. Standard-to-substrate synthetic routes enable the conversion of fluoroarene-appended pharmaceutical agents to their [18F]radiofluorination substrates without the need to carry out a lengthy multi-step synthesis of precursor. Herein, we report the optimization of a previously reported nickel-catalyzed defluoroboronation reaction, where replacement of an air-sensitive Ni(0) catalyst with Ni(II)trifluoromethanesulfonate (Ni(OTf)2) enables the preparation of Bpin precursors without the need for specialized equipment, such as a glovebox. In addition, this method can also be used to produce analogous arylstannanes, which are an alternate [18F]fluorination precursor species [2].
Methods: All reagents were used as received from commercial suppliers. Defluoroborylation conditions reported by Martin et al. were the starting point for further optimization, albeit with Ni(OTf)2 instead of Ni(COD)2 as catalyst and the reportedly inactive bis(pinacolato)diboron (Bpin2) as the boron source instead of bis(neopentyl)diboron. Hexabutylditin (Sn2Bu6) was used as the tin source in defluorostannylation reactions. Optimization of reaction parameters was carried out in 4 mL sealed borosilicate vials at a 0.05 mmol scale (w.r.t 4-fluoroanisole substrate). No attempts were made to reduce air/moisture exposure during reaction preparation beyond typical chemical handling procedures. Vials were sealed with a PTFE cap and held at 110 ⁰C without stirring for 18 hours. Thin layer chromatography and analytical HPLC (Luna C18, 5µ, 4.6x150mm; MeCN/H2O (0.1% TFA) gradient) were then used to determine % yield of 4Bpin-anisole product.
Results: The following reactant loading conditions provided the best %yield: 4-fluoroanisole (0.05 mmol, 1 eq), Ni(OTf)2 (0.2 eq), tricyclohexylphosphine (0.2 eq), sodium phenoxide (3 eq), Bpin2 (3 eq), in 1 mL solvent. Other phosphorous- and nitrogen-based ligands were tested but were found to be inferior to PCy3. Addition of a chloride ion source (LiCl or TBACl) did not affect yield. MTBE solvent led to the greatest yield (18.3%; HPLC yield), although product formation was also observed with THF, 1,4-dioxane, and toluene as solvents. An isolated yield of 17% was obtained following work-up and column chromatographic purification, and 1H-NMR was used to confirm identity via comparison with the spectrum of commercially available 4-Bpin-anisole. Formation of 4-SnBu3-anisole was qualitatively observed with TLC when Bpin2 was replaced with Sn2Bu6.
Conclusion: This work demonstrates proof-of-principle for an operationally simple method to synthesize boron and tin [18F]fluorination precursors from an electron-rich fluoroarenes in one step and in low but reasonable yield. Effort is currently underway to further optimize this process and increase % yield of the boron and tin products and to determine the overall scope of this method. References [1] Mossine, A. V. et. al. Org. Lett., 2015, 17, 5780-5783; [2] Makaravage, K. J. Org. Lett. 2016, 18, 5440-5443. Research Support: We acknowledge the NIH (R01EB021155 to M.S.S. and P.J.H.S.), US DOE/NIBIB (DE-SC0012484 to P.J.H.S.), and Merck (M.S.S. and P.J.H.S.) for financial support.