Abstract
276
Objectives: Tropane derivatives are well established 123I-ligands for SPECT/CT imaging of dopamine transporter (DAT) in Parkinson disease (PD) and Lewy body dementia (LBD). Nevertheless, several 11C/18F radioligands have been developed for PET imaging of DAT, with the expectation of an earlier detection of dopaminergic denervation, due to higher resolving images properties and better quantification of PET. We report here the first in-human PET evaluation of a novel tropane-based DAT ligand, namely 18F-LBT999.
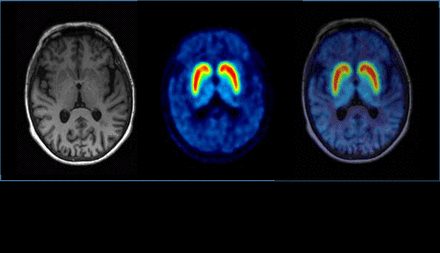
Methods: After intravenous bolus injection of 18F-LBT999 (3.6 MBq/kg), 8 healthy subjects (age: 69±9y) underwent a 90min dynamic PET scan with an Ingenuity Philips tomograph. Venous samples were concomitantly obtained for metabolite analysis. Time activity curves (TACs) were generated for several ROIs (caudate (Cd), putamen (Pu), occipital cortex, substantia nigra and cerebellum (Cb)), defined over each T1 3D MRI with Pmod® Software. Cerebellum was used as a reference region to calculate binding potentials (BP).
Results: There were no adverse events or clinically detectable pharmacologic effects reported. 18F-LBT999 PET revealed a good cerebral distribution of radioactivity, especially with an intense and symmetric radiotracer uptake in both putamen and caudate (BP of 6.3±1.1 and 6.8±1.1, respectively), without other brain abnormal tracer accumulation, and lowest activity in the Cb. Regional TACs showed a maximal uptake for all ROIs 7 min pi, followed by a plateau until the end of PET acquisition for both Cd and Pu, whereas other ROIs exhibited a two phases washout of activity: a first phase of rapid decrease between 7 and 35 min, followed by a slower one from 35 min until the end of PET acquisition. The parent fraction of 18F-LBT999 in plasma was 80%, 60% and 40% at 15, 30 and 45 min pi, respectively.
Conclusion: Time stability of BP in both Cd and Pu, together with the evidence of admissible in vivo metabolism, suggest an optimal image acquisition for 10 min between 30 and 40 min pi (Figure 1). These preliminary findings support the usefulness of 18F-LBT999 for a quantitative evaluation of presynaptic dopaminergic neurons impairment in clinical setting. Research Support: This study was supported by the French National Agency for Research (‘‘Investissements d’Avenir’’ no. ANR-11-LABX-0018-01), IRON by ITMO-INSERM, and by Cyclopharma Laboratories.