Abstract
10
Objectives: Sphingosine-1-phosphate receptor 1 (S1P1) is a validated target for PET imaging of neuroinflammation and for therapeutic intervention. Based on our previous effort on development of PET radiotracers for imaging S1P1 [1], here we report our recent work on optimization of the lead structure and further evaluation in an animal model of neuroinflammation. The goal of this work is to identify a clinical suitable PET tracer for imaging S1P1 expression in central nervous system (CNS) with appropriate tracer kinetics.
Methods: We explored new analogs by incorporating polyethylene glycol (PEG) chains into the structure of the lead compound TZ35104. The binding affinity and specificity of new-synthesized S1P1 compounds were determined by radioligand competitive binding assays. Three potent S1P1 ligands were radiolabeled with fluorine-18 using a one-pot two-step procedure: 1) nucleophilic substitution of the tosylate precursor with dried K[18F]/F-; 2) acidolysis of the methoxymethyl protecting group with 6.0 M HCl. MicroPET imaging studies of each radiotracer were performed in normal nonhuman primates (NHPs), the experimental autoimmune encephalomyelitis (EAE) rat model and sham control rats. A Focus 220 scanner was used for microPET studies in male cynomolgus macaques (8 - 9 kg). An Inveon PET/CT system was used for microPET studies in female Lewis rats (~150 g) that received immunization with myelin basic protein (MBP) and had severe neurological symptoms of EAE; upregulated S1P1 protein expression in lumbar spinal cord of EAE rats had been detected [2].
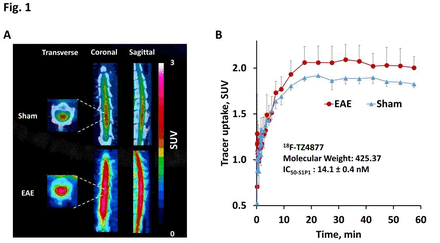
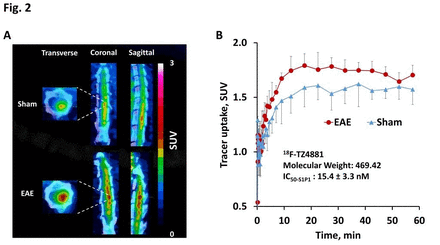
Results: TZ4877 and TZ4881 have high binding affinity for S1P1 with IC50-S1P1 values of 14.1 ± 0.4 nM and 15.4 ± 3.3 nM respectively, and good selectivity (> 100-fold for S1P1 versus S1P2/3). Radiosyntheses of 18F-TZ35104, 18F-TZ4877 and 18F-TZ4881 were achieved with 40 ± 5% radiochemical yield and high specific activity (> 45 GBq/µmol, decay corrected to the end of synthesis). NHP microPET scans revealed that all three radioligands penetrated the brain and had high tracer accumulation. Rodent microPET studies using 18F-TZ4877 and 18F-TZ4881 revealed approximately 10% increase of tracer uptake (standardized uptake values (SUVs), P < 0.05, n = 3 - 4) in EAE rat lumbar spinal cord compared to shams. Compared to 18F-TZ35104 [1], both of 18F-TZ4877 and 18F-TZ4881 retained elevated tracer uptake in EAE rat spinal cord.
Conclusion: Both of 18F-TZ4877 and 18F-TZ4881 were able to detect increased S1P1 levels in response to neuroinflammation in the EAE rat model. These 3 radiopharmaceuticals (18F-TZ4877, 18F-TZ4881 and 18F-TZ35104) have different tracer kinetics and binding profiles, but each could be implemented for imaging neuroinflammation in different conditions. Further characterization in NHP models of neuroinflammation and tracer metabolism studies are warranted for selecting the suitable tracer for clinical applications in humans. Research Support: DESC0008432, DESC0012737, NS075527, NIMH092797. References: 1. Rosenberg et al. J Nucl Med. 2016;57:1; 2. Liu et al. Mol Imaging Biol. 2016; 18:724-32. Fig. 1 MicroPET studies of EAE rat spinal cord using the S1P1-specific radioligand, 18F-TZ4877. A) Summed microPET/CT images showing 18F-TZ4877 had higher accumulation in EAE rat lumbar spinal cord than sham; B) Tissue activity curves revealed 9.0 % increase of tracer uptake (standardized uptake values (SUVs), P < 0.05, n = 3 - 4) in EAE rats compared with shams. Fig. 2 MicroPET studies of EAE rat spinal cord using the S1P1-specific radioligand, 18F-TZ4881. A) Summed microPET/CT images showing 18F-TZ4881 had higher accumulation in EAE rat lumbar spinal cord than sham; B) Tissue activity curves revealed 10.3 % increase of tracer uptake (SUVs, P < 0.05, n = 3) in EAE rats compared with shams.