Abstract
71
Objectives Cognitive impairment of Parkinson’s disease, commonly in executive domain, has clinical importance due to significant influence on daily life functioning of patients. According to the caudate-frontal loop theory, dysfunction of caudate nucleus, connected with medial orbitofrontal cortex, contributes to the impairment of executive function, whereas putamen, connected with the supplementary motor cortex, is related to motor function [1]. Here, using dual time F-18 FP-CIT, we evaluate cortical and striatal uptake pattern simultaneously in PD patients with impaired executive function, finding evidence for neural correlates between caudate and frontal cortex.
Methods 108 patients with PD, divided into younger and older groups, were enrolled in this study (table 1). PD patients underwent the Seoul Neuropsychological Screening Battery : a stroop test for executive function, a digit span for attention, a Boston naming test for language, the Rey complex figure test for visuospatial, the Seoul verbal learning test for verbal memory (Z-score < 1.5 SD : abnormal) [2]. Patients were divided into 4 groups: group A with 26 healthy controls, group B with impaired motor and executive function, group C with impaired motor and cognition other than executive domain, group D with impaired motor only. H & Y stage, UPDRS III motor did not differ between groups (ANOVA) (table 1). F-18 FP-CIT PET/CT was acquired at 10 minutes and 2 hour. Cortical perfusion in early image were compared between groups using SPM analysis (ANOVA). In delayed image, SUVmean of both caudate, putamen and cerebellum were measured using MRI and PET N30R83 VOI based Hammers atlas method in PMOD version 3.7.0. Specific binding uptake was defined as follows: (Striatal SUVmean - cerebellar SUVmean) / cerebellar SUVmean. Difference of striatal uptake between groups was compared using both VOI and SPM analysis (t-test).
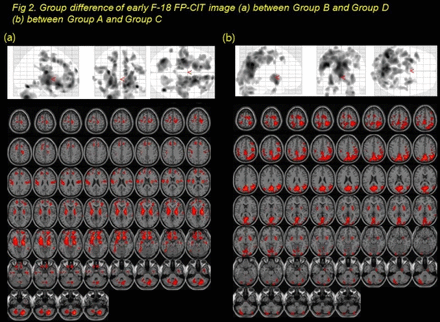
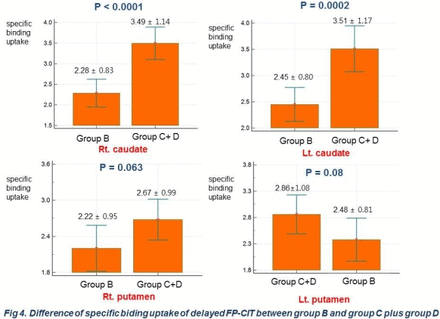
Results In early CIT, both superior, middle, medial orbitofrontal, temporal, lentiform nucleus, temporal, cerebellum showed difference between group A and B (p< 0.05, k=100, Fig 1a). Both lentiform nucleus, midbrain,cerebellum, superior frontal, precentral, temporal showed difference between group A and group D (Fig 1b), suggesting PD-related motor pattern. Comparing group B with D, there were different mainly in both superior, middle, medial orbitofrontal areas governing executive function assessed in stroop test, suggesting PD-related cognitive(executive) pattern (Fig.2a). Both occipital, temporal, parietal, posterior cingulate, lentiform nucleus showed difference between group A and C (Fig 2b). Areas showing decreased perfusion in early CIT image correspond to cognitive domain impaired in neuropsychological test. In delayed image, specific binding uptakes were different in both caudate (R: p = 0.0003, L: p = 0.0108), but not in both putamen (R: p= 0.1916, L: p= 0.738) between group B and D (Fig 3). Specific binding uptakes were different in both caudate (R: p = 0.0001, L: p < 0.0002), but not in both putamen (R: p = 0.06, L: p =0.08) between group B and group C plus D (Fig 4). In SPM analysis, there was difference between group B and group D in both caudate (Fig 5, t-test, p < 0.05, k = 100), not in both putamen, which correlates well with the results of VOI analysis.
Conclusions By evaluating PD-specific cognitive cortical perfusion and striatal specific uptake pattern using dual time F-18 FP-CIT, we provides supporting evidence for neural correlates between caudate and frontal cortex. Decreased dopaminergic function of caudate nucleus, not putamen, is related to impaired performance in executive function, which is related to frontal lobe function. Also, caudate hypofunction is specific to frontal executive domain, not related with other cognitive domains. Hence, dual time F-18 FP-CIT is useful for integrative evaluation of both cognitive and motor impairment of PD patients in clinical settings.
Table 1. patient information