Abstract
Our goal is to develop minimally invasive biomarkers for predicting radiation-induced lung injury before symptoms develop. Currently, there are no biomarkers that can predict radiation pneumonitis. Radiation damage to the whole lung is a serious risk in nuclear accidents or in radiologic terrorism. Our previous studies have shown that a single dose of 15 Gy of x-rays to the thorax causes severe pneumonitis in rats by 6–8 wk. We have also developed a mitigator for radiation pneumonitis and fibrosis that can be started as late as 5 wk after radiation. Methods: We used 2 functional SPECT probes in vivo in irradiated rat lungs. Regional pulmonary perfusion was measured by injection of 99mTc-macroaggregated albumin. Perfused volume was determined by comparing the volume of distribution of 99mTc-macroaggregated albumin to the anatomic lung volume obtained by small-animal CT. A second probe, 99mTc-labeled Duramycin, which binds to apoptotic cells, was used to measure pulmonary cell death in the same rat model. Results: The perfused volume of lung was decreased by about 25% at 1, 2, and 3 wk after receipt of 15 Gy, and 99mTc-Duramycin uptake was more than doubled at 2 and 3 wk. There was no change in body weight, breathing rate, or lung histology between irradiated and nonirradiated rats at these times. Pulmonary vascular resistance and vascular permeability measured in isolated perfused lungs ex vivo increased at 2 wk after 15 Gy of irradiation. Conclusion: Our results suggest that SPECT biomarkers have the potential to predict radiation injury to the lungs before substantial functional or histologic damage is observed. Early prediction of radiation pneumonitis in time to initiate mitigation will benefit those exposed to radiation in the context of therapy, accidents, or terrorism.
Injury by ionizing radiation to the lung manifests in two phases (1,2). The first phase (pneumonitis) occurs 6–12 wk after irradiation. The second phase (pulmonary fibrosis) develops over the months to years after exposure (1,2). Our goal is to develop biomarkers that can predict severe lung injury before symptoms manifest and in time for mitigating agents to be applied. This goal is relevant to radiotherapy and to the National Institutes of Health program to develop countermeasures for nuclear accidents and radiologic terrorism. Using preclinical models, we have mitigated radiation pneumonitis and fibrosis with an angiotensin-converting enzyme inhibitor, enalapril, started 5 wk after irradiation (3). We are now developing noninvasive biomarkers to detect severe lung injury before this 5-wk time point.
Because there is a latent period between irradiation and symptoms of injury to the lung, biomarkers can be measured in this time to predict outcomes. Changes in regional perfusion by localized radiation but not to the whole lung have been detected by SPECT in vivo by injection of 99mTc-macroaggregated albumin (MAA) (4–7). The albumin is lodged within the intricate microvasculature of the lung in proportion to flow and detected by the γ-camera of the SPECT system. In general, perfusion redistribution from radiation is more frequently reported than ventilation defects (4,8), and both are more sensitive than changes in lung density identified with CT (4).
A different SPECT probe, 99mTc-labeled tetracycline hydrochloride (Duramycin; Durvet, Inc.), is known to have high affinity and specificity for phosphatidylethanolamine, which is externalized in apoptotic and other dying cells (9). Because radiation has been reported to induce apoptosis (10–12), necrosis, and mitotic cell death, this marker was used to assess whole-body tissue damage after radiation exposure (13). We tested the two probes 99mTc-MAA and 99mTc-Duramycin as candidate biomarkers to monitor injury to the lungs at 1–4 wk after irradiation. We followed our imaging studies with histologic evaluation and other methods to confirm and interpret our findings.
MATERIALS AND METHODS
Animal Care
All animal protocols and euthanasia criteria have been previously described (3,14) and were approved by the Institutional Animal Care and Use Committee. A separate group of animals was used to measure each endpoint.
Irradiation
Unanesthetized female WAG/RijCmcr rats were irradiated with 15 Gy to only the thorax at 9–10 wk of age as previously described (15). They were studied for noninvasive or terminal endpoints 1, 2, 3, and 4 wk after irradiation. Nonirradiated rats were not sham-irradiated but were of the same age, strain, and sex and maintained in parallel with each batch of irradiated rats.
SPECT/CT
MAA (Jubilant DraxImage) was labeled with 99mTc according to the kit instructions, and Duramycin (3,035 g/mole molecular weight) was prepared and labeled as previously described (16,17). One group was then administered a single dose of 24.6 ± 3.9 MBq (mean ± SD) of 99mTc-MAA via the tail vein using a 25-gauge catheter. Another group received a tail vein injection of 38.5 ± 7.6 MBq of 99mTc-Duramycin. The animals were positioned in a SPECT/CT scanner (Triumph; TriFoil Imaging) and first underwent rapid CT scanning for anatomic localization. At 5 min (for rats given 99mTc-MAA) or 50 min (for rats given 99mTc-Duramycin) after injection, that is, times for optimal lung uptake, in vivo radionuclide imaging was performed using multipinhole collimation with two γ-head detectors, a 130- to 150-keV energy window, and acquisition of 72 projections for 10 s each. SPECT and CT data were reconstructed and coregistered using inbuilt software (13).
Image Analysis
The reconstructed CT and 99mTc-MAA image volumes were segmented and analyzed to determine the perfused lung volume as follows. First, the lung region within the CT image volume was identified as previously described (18). A grayscale window with a lower threshold of zero (corresponding to air) and an upper threshold of the maximum grayscale value within the lung parenchyma region, excluding major blood-filled vessels, was established and used to determine the boundaries of the anatomic lung region. The total number of voxels within this lung region was then scaled by the volume of each voxel to determine the anatomic lung volume. This region served as a binary lung mask that was then applied to the reconstructed 99mTc-MAA image volume. The total number of nonzero voxels within the 99mTc-MAA SPECT lung region was determined and scaled by the volume of each SPECT voxel to determine the perfused lung volume. Finally, the fraction of lung perfused was determined by dividing the perfused lung volume obtained from 99mTc-MAA by the anatomic lung volume obtained from CT. Perfused fractions were infrequently greater than 1, since the volume of a voxel differed between SPECT and CT.
For experiments involving 99mTc-Duramycin, the reconstructed CT and SPECT image volumes were coregistered, and lung boundaries obtained from CT were applied as a mask to determine the 99mTc-Duramycin lung region as described for 99mTc-MAA. 99mTc-Duramycin lung uptake was then determined as average 99mTc-Duramycin counts per voxel within the lung region normalized to injected dose (16,17,19).
Breathing Rate
Breathing rates and body weights were measured serially in an independent group of rats as described previously (14,20).
Isolated Perfused Lung Preparation and Pulmonary Vascular Endothelial Filtration Coefficient (Kf)
The heart and lungs from an independent group of rats were isolated at the 2-wk time point and suspended from a calibrated force displacement transducer (model FT03; Grass Instruments), and lung weight was monitored continuously as previously described (21). The lungs were perfused and ventilated (40 breaths/min) with end-inspiratory and end-expiratory pressures of about 8 and 4 cm H2O. The Kf was determined using the approach described by Bongard et al. (22). The venous pressure was set at atmospheric pressure and then raised to 5 cm H2O for 10 min and 13.5 cm H2O for an additional 10 min. At the end, the lungs were removed from the perfusion system, the arterial and venous cannulas connected, and the pressure drop in the cannulas (ΔPcan) determined. The pulmonary vascular pressure, RV, was then calculated using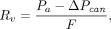 where Pa is the pulmonary arterial pressure measured at the end of the 10-min stabilization period with venous pressure set at 0 mm Hg, and F is the pump flow rate (0.03 mL/min/g of body weight).
where Pa is the pulmonary arterial pressure measured at the end of the 10-min stabilization period with venous pressure set at 0 mm Hg, and F is the pump flow rate (0.03 mL/min/g of body weight).
Lung Wet/Dry Weight
After the Kf studies were completed, the lungs were weighed, dried at 60°C–80°C for 24 h, and weighed again to calculate the wet and dry weights.
Histology
Whole-mount sections of the left lung were stained with hematoxylin and eosin (Richard-Allan), with antibodies to cleaved caspase 3 (CP229B; Biocare (17)) to detect apoptotic cells (23), or with CD68 (MCA341R; abD Serotec) to identify macrophages (24).
Histologic scores were obtained in hematoxylin- and eosin-stained lung sections for the following 4 endpoints, as previously described (14,20): vascular wall thickness, foamy macrophages, CD68+ macrophages, and alveolar wall thickness. Caspase 3–positive cells were counted as previously described (17), and CD68+ macrophages (24) were counted in 5 fields randomly selected from corresponding areas in each section to cover the whole mount.
Statistical Analysis
Data were analyzed using StatView (version 5.0.1; SAS Institute) and expressed as mean ± SD or median ± 75% and 25% ranges if normality or equal variance tests failed. For some tests, the control values at 1 and 4 wk were pooled if they were not statistically different. For multigroup comparisons, the significance of differences was assessed by ANOVA with the Holm-Šidák or Dunn methods and comparisons were made to the nonirradiated controls. For 2-group comparisons, t tests were used or Mann–Whitney tests if normality tests failed.
RESULTS
Body Weight After Radiation to Thorax Only
Nonirradiated animals gained weight in the first week (2.7 ± 2.4 g), but irradiated rats lost weight (−1.6 ± 4.2 g, P = 0.041, n = 5/group). There was no difference in weight gain or loss between the groups at 2, 3, or 4 wk.
Breathing Rate After Radiation
The respective breathing rates at 1, 2, 3, and 4 wk were 99.4 ± 4.3, 113.0 ± 10.4, 104.6 ± 7.9, and 102.8 ± 8.9 breaths/min in nonirradiated controls and did not differ from corresponding values after 15 Gy: 103.2 ± 4.3, 108.8 ± 6.3, 108.8 ± 9.4, and 98.4 ± 4.0 breaths/min (n = 5/group).
Decrease in Perfused Lung Volume with 99mTc-MAA SPECT After Radiation
Figure 1 shows representative transaxial slices from the 99mTc-MAA (left) and 99mTc-Duramycin (right) reconstructed volume of a nonirradiated control rat (top) and a rat 2 wk after receiving 15 Gy of irradiation (bottom). Fractional perfused lung volume was determined from the 99mTc-MAA images as described in the Materials and Methods section at 1 and 4 wk in nonirradiated controls. Because there was no difference between these groups, they were combined and compared with values obtained at 1, 2, 3, and 4 wk after 15 Gy. Figure 2 shows all values after normalization to the median value of the controls. There was a decrease in perfused lung volume starting as early as 1 wk (18%) and up to 3 wk (27%) after irradiation as compared with nonirradiated controls.
Typical 99mTc-MAA (left) and 99mTc-Duramycin (right) transaxial slices from reconstructed volumes obtained from control (top) and 15-Gy–irradiated (bottom) rats. Color images represent segmented SPECT lung region coregistered with gray-scale CT images.
Decrease in perfused pulmonary volume by radiation, as determined by ratio of 99mTc-MAA SPECT perfused volume to coregistered CT anatomic volume. Line is median value in nonirradiated rats (no rads; n = 12) and irradiated rats at 1 wk (n = 8), 2 wk (n = 10), 3 wk (n = 13), and 4 wk (n = 9) after 15 Gy to thorax. *P < 0.05 vs. nonirradiated controls.
Increase in 99mTc-Duramycin Uptake After Radiation
The 99mTc-Duramycin images of Figure 1 reveal very little uptake in the control lung, consistent with our previous results (16,17), but enhanced uptake was seen 2 wk after irradiation. Figure 3 reports average 99mTc-Duramycin counts per voxel within the lung region for each rat. There was no change in 99mTc-Duramycin uptake at 1 wk after irradiation but a greater than 100% increase was observed at 2 and 3 wk compared with nonirradiated controls.
Increase in 99mTc-Duramycin lung uptake in irradiated lungs vs. controls. Line is median value in nonirradiated rats (no rads; n = 12) and irradiated rats at 1 wk (n = 5), 2 wk (n = 11), and 3 wk (n = 5) after 15 Gy to thorax. *P < 0.05 vs. control. **P < 0.05 vs. 15 Gy at 1 wk.
Increase in Apoptotic Cells in Lungs After Radiation
Figure 4 shows the number of caspase 3–positive apoptotic cells per field in rat lungs. Similar to the 99mTc-Duramycin uptake in Figure 3, there was no increase in apoptotic cells after 1 wk in irradiated rats as compared with nonirradiated controls, but there was an increase in the median number of apoptotic cells per lung field at 2, 3, and 4 wk after irradiation.
Increase in apoptotic cells in lung as determined by cleaved caspase 3+ cells using immunohistochemistry. Apoptotic cells per field in nonirradiated and irradiated rats are shown at 1, 2, 3, and 4 wk after 15 Gy to thorax. Line is median value in nonirradiated rats (no rads; n = 15) and irradiated rats (n = 8 per group). *P < 0.05 vs. control.
Histologic Changes in Lungs at 4 but Not at 1, 2, or 3 Weeks After Radiation
We measured key histologic markers of radiation injury in nonirradiated and irradiated rat lungs (14,20): vessel wall thickness, alveolar wall thickness, and macrophage counts. The values for these markers did not change in nonirradiated controls at ages corresponding to 1 and 4 wk after irradiation. There was no difference in vessel wall thickness between irradiated rats at 1, 2, 3, or 4 wk and pooled controls at 1 and 4 wk. Although the median score for alveolar wall thickness (Fig. 5D) and macrophage count (Fig. 5E) did not change at 1, 2, or 3 wk, both values were higher at 4 wk after 15 Gy (Figs. 5D and 5E). Similar results were observed for CD68+ macrophages measured at 2 and 4 wk after 15 Gy (Fig. 5F).
Histologic analysis showing increase in alveolar wall thickness and macrophages after 15 Gy of whole-thorax irradiation. Representative fields are shown of hematoxylin- and eosin-stained lung sections from nonirradiated lung (A), lung 2 wk after 15 Gy (B), and lung 4 wk after 15 Gy (C). Solid arrow points to macrophages, dashed arrow to thickened alveolar wall. (D) Scores for alveolar wall thickness. (E) Counts for foamy macrophages. (F) Counts for CD68+ macrophages. Line is median value in nonirradiated rats (n = 16 each for D and E and 8 for F) and irradiated rats (n = 8 each for D, E, and F). *P < 0.05 vs. control at 4 wk.
Increase in Pulmonary Vascular Permeability and Resistance at 2 Weeks After Radiation
We tested the permeability and resistance of lung vessels 2 wk after irradiation, a time point when both 99mTc-MAA distribution and 99mTc-Duramycin uptake were different from nonirradiated controls. Ex vivo pulmonary vascular resistance (Torr/mL/min) increased from 0.89 ± 0.14 in nonirradiated lungs to 1.17 ± 0.19 in irradiated lungs after 2 wk (n = 5, P = 0.028) (Fig. 6, top). The pulmonary Kf (mL/min/cm H2O/g of dry lung weight) was increased by radiation from 0.017 ± 0.005 in nonirradiated lungs to 0.050 ± 0.010 in matched irradiated lungs at the 2-wk time point (n = 5, P < 0.001) (Fig. 6, bottom). The wet-to-dry weight ratio of the perfused lungs showed an increase from 5.9 ± 0.4 in controls to 7.0 ± 0.2 after irradiation. The mean ± SD for the wet weight after perfusion at similar pressures increased from 0.81 ± 0.07 g in nonirradiated controls to 1.16 ± 0.09 g, an increase of 42%.
Increase in pulmonary vascular resistance and permeability (Kf) in isolated perfused lungs 2 wk after irradiation with 15 Gy. Line is mean value (n = 5 rats per group). No rads = nonirradiated rats. *P < 0.05 (top) or < 0.001 (bottom) vs. control.
DISCUSSION
SPECT has made it possible to develop customized physiologic probes for noninvasive imaging. We have used two probes labeled with 99mTc to detect radiation-induced preclinical vascular changes and increased apoptosis, two endpoints known to be altered by radiation. Characterization of these changes has the potential to define biomarkers for in vivo detection of radiation injury to the lungs. The first probe, 99mTc-MAA, with a particle size of 10–40 μm, lodges in pulmonary capillaries in proportion to blood flow (18). 99mTc-MAA is in common clinical use for detection of pulmonary embolism. The second probe is the antibiotic Duramycin, which is a small molecule that binds to apoptotic cells (9,13) and has been labeled with 99mTc for SPECT imaging (9,13). There was an approximately 20% decrease in the perfused volume of the lung as early as 1 wk after irradiation with 15 Gy to both lungs, which was sustained for up to 3 wk. These data match our observation of increased pulmonary vascular resistance in ex vivo perfused lungs. The uptake of 99mTc-Duramycin in the thorax more than doubled at 2 and 3 wk but was not increased at 1 wk after irradiation. Further studies of 99mTc-Duramycin uptake from only perfused voxels of the lung may determine whether there is a correspondingly higher increase in perfused areas than in the whole lung.
Our data provide strong evidence of preclinical radiation-induced vascular injury in the absence of histologic changes in alveolar wall thickness and macrophage counts. Macrophages (index of inflammatory state) were counted in hematoxylin- and eosin-stained sections, and we confirmed with more specific CD68 staining that they were not increased at 2 wk, when perfusion and apoptosis were already altered. Though no increase in vascular wall thickness or occlusion of vascular lumen was noted histologically for up to 4 wk, there was an increase in vascular permeability, Kf, and vascular resistance at 2 wk, when measured in irradiated perfused lungs ex vivo. The increase in permeability was also reflected by an increase in wet-to-dry lung weight after perfusion. A dose-dependent increase in vascular permeability at sacrifice has previously been reported after 2 wk in rats exposed to 5–40 Gy to one lung only (25). Molteni et al. also described subendothelial and perivascular edema 1 d after hemithorax irradiation in rats (26). They reported segmental separation of the endothelium from the basement membrane. In another study, in vivo lung vascular permeability was doubled at 2 wk after whole-thorax irradiation (similar to our results) and peaked to 3 times the normal value by 3 wk (27). Increased permeability in our model may be pathophysiologically related to apoptosis of endothelial cells, as has been reported for other insults (28). These studies by us and others demonstrate that the vasculature in the lungs is injured in the first 4 wk after irradiation to the whole thorax without manifesting clinical symptoms or histologic changes. The results imply that roentgenographic evidence of pulmonary edema in a patient after possible or known radiation exposure could be a prelude to clinical radiation pneumonitis. Such patients may also be good candidates for SPECT imaging to detect endothelial apoptosis.
99mTc-Duramycin is a recently developed biomarker for SPECT cell death imaging. It has several desirable imaging properties; it binds with very high affinity (the dissociation constant is in the low nanomolar range (29,30)) and high specificity for membrane-bound phosphatidylethanolamine (31), wherein the binding is stabilized by ionic interaction. Moreover, 99mTc-Duramycin has a low molecular weight (3,035 g/mole) and is rapidly cleared from the blood (half-life < 4 min (17,32)), thereby keeping background levels low.
In this study, lung uptake of 99mTc-Duramycin was increased (∼160%) at 2 and 3 wk after irradiation, a finding that was supported by the increase in cleaved caspase 3+ apoptotic cells. This observation is consistent with a previous study using a hyperoxic lung injury model in which 99mTc-Duramycin lung uptake was shown to correlate strongly with cleaved caspase 3+ apoptotic endothelial cells (17). Though the increase in vascular permeability could contribute to an increase in 99mTc-Duramycin lung signal, the high affinity and specificity of binding of 99mTc-Duramycin to apoptotic cells, the rapid clearance from the blood, and the increase in cleaved caspase 3 in irradiated rats strongly suggest that the increase in the 99mTc-Duramycin was due to increased cell death, though further experiments are needed to verify this possibility. Despite these potential limitations, the fact that the increased 99mTc-Duramycin uptake was much higher than the degree of vascular leakage suggests that increased 99mTc-Duramycin uptake may serve as an early biomarker of radiation-induced lung injury.
A small increase in perfusion was recorded 4–6 wk (the earliest time point of the study) after 25 Gy to one lung in Sprague–Dawley rats (7). These measurements were done using 99mTc-MAA and analyzed by comparing perfusion between the irradiated lung and the nonirradiated lung of the same animal. Perfusion in the irradiated lung was lower between 6 and 30 wk (7). A decrease in perfusion to an irradiated lung was recorded in Fischer rats 3 d after 28 Gy; perfusion recovered by 2 wk and then gradually declined to as low as 15% by 10 wk (6). Our results are difficult to compare with these studies since we irradiated both lungs and are unique in describing a redistribution of 99mTc-MAA from as early as 1 wk and up to at least 3 wk after irradiation. Changes in perfusion distribution may be explained by the changes we measured in vascular resistance. In addition, lung arterioles exhibit decreased ability to constrict or dilate to physiologic stimuli after irradiation (33,34). Such an endpoint cannot be measured in vivo and may contribute to the changes in perfusion we detected by 99mTc-MAA. Experimentation to test the multiple and known effects of radiation on pulmonary arterioles toward the SPECT signals will take considerable time and resources. Our first goal is to apply our findings to the rapid development of biomarkers for radiation injury to the lungs.
Use of 99mTc-Duramycin for whole-body imaging of irradiation-induced tissue damage has been examined 72 h after 15 Gy to the total body of a rat (13). There was increased uptake in the gut, bone, and thymus at this early time. Injury to the lung occurs later (1–3). Our SPECT results using 99mTc-Duramycin suggest ongoing apoptosis in the lung beginning around 2 wk after irradiation and continuing (Fig. 3). The pattern of change in signal was similar in imaging and histology, increasing from 2 wk after irradiation but not at 1 wk (Figs. 3 and 4). These results are important in that they imply patients with possibly injurious exposure could be screened as early as 2 wk after exposure. Those with increased lung apoptosis would be excellent candidates for treatment with agents such as angiotensin-converting enzyme inhibitors, which mitigate lung injury when given up to 5 wk after exposure (3).
CONCLUSION
Our findings of decreased perfused volume and increased 99mTc-Duramycin uptake in the lungs of irradiated animals are novel and could be developed into new methods of predicting lung injury by radiation even before substantial functional or histologic damage is observed. Further studies with different doses of radiation will determine the specificity for determining lethal lung injury before symptoms develop.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was funded by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (1R01AI101898 and U01AI107305-01) and by the National Institutes of Health/National Heart Lung and Blood Institute (HL116530, HL120209, 1I01BX001681, and R15HL129209). No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Dr. John E. Moulder for helpful discussions and for critically reading the manuscript. Histologic work was performed by the Pediatric Biobank and Analytic Tissue Core.
Footnotes
Published online Mar. 31, 2016.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication May 13, 2015.
- Accepted for publication January 21, 2016.













