Abstract
Abnormalities of zinc homeostasis are indicated in many human diseases. A noninvasive imaging method for monitoring zinc in the body would be useful to understand zinc dynamics in health and disease. To provide a PET imaging agent for zinc, we have investigated production of 63Zn (half-life, 38.5 min) via the 63Cu(p,n)63Zn reaction using isotopically enriched solutions of 63Cu-copper nitrate. A solution target was used for rapid isolation of the 63Zn radioisotope from the parent 63Cu ions. Initial biologic evaluation was performed by biodistribution and PET imaging in normal mice. Methods: To produce 63Zn, solutions of 63Cu-copper nitrate in dilute nitric acid were irradiated by 14-MeV protons in a low-energy cyclotron. An automated module was used to purify 63Zn from 63Cu in the target solution. The 63Cu–63Zn mixture was trapped on a cation-exchange resin and rinsed with water, and the 63Zn was eluted using 0.05 N HCl in 90% acetone. The resulting solution was neutralized with NaHCO3, and the 63Zn was then trapped on a carboxymethyl cartridge, washed with water, and eluted with isotonic 4% sodium citrate. Standard quality control tests were performed on the product according to current good manufacturing practice, including radionuclidic identity and purity, and measurement of nonradioactive Zn+2, Cu+2, Fe+3, and Ni+2 by ion-chromatography high-performance liquid chromatography. Biodistribution and PET imaging studies were performed in B6.SJL mice after intravenous administration of 63Zn-zinc citrate. 63Cu target material was recycled by eluting the initial resin with 4N HNO3. Results: Yields of 1.07 ± 0.22 GBq (uncorrected at 30–36 min after end of bombardment) of 63Zn-zinc citrate were obtained with a 1.23 M 63Cu-copper nitrate solution. Radionuclidic purity was greater than 99.9%, with copper content lower than 3 μg/batch. Specific activities were 41.2 ± 18.1 MBq/μg (uncorrected) for the 63Zn product. PET and biodistribution studies in mice at 60 min showed expected high uptake in the pancreas (standard uptake value, 8.8 ± 3.2), liver (6.0 ± 1.9), upper intestine (4.7 ± 2.1), and kidney (4.2 ± 1.3). Conclusion: A practical and current good manufacturing practice–compliant preparation of radionuclidically pure 63Zn-zinc citrate has been developed that will enable PET imaging studies in animal and human studies. 63Zn-zinc citrate showed the expected biodistribution in mice.
Zinc is an essential metal in the body that is at the reactive center of more than 300 metabolic enzymes and has fundamental roles in protein structure and protein–protein interactions (1). It participates in the tertiary, quaternary, and quinary structure of proteins; in protein aggregation; and in the structure of protein domains for interactions with other proteins, DNA/RNA, and lipids. Zinc deficiency affects about 2 billion people in the developing world (2), whereas excess zinc consumption can also cause detrimental effects of ataxia, lethargy, and copper deficiency. Disruption of zinc homeostasis may be involved in metabolic syndrome, diabetes, and diabetic complications (3). Zinc homeostasis may play an important role in certain cancer types, including pancreatic cancer (4), prostate cancer (5), and breast cancer (6). Also, zinc is associated with the aggregation of β-amyloid proteins that accumulate in the brains of patients with Alzheimer disease (AD) (7). Metal chelation therapy is under investigation for treatment of AD with the intent of altering zinc and copper binding within β-amyloid deposits in the brain (8). Clearly, a noninvasive method to measure zinc dynamics in the body would be of high interest for understanding the pathophysiology of a broad range of diseases. It may also be useful for monitoring therapies that are directed at changing zinc homeostasis.
There are 3 positron-emitting isotopes of zinc that have potential to be used as PET biomarkers of zinc kinetics in living systems: 62Zn, 63Zn, and 65Zn (Table 1). 62Zn (half-life [T1/2], 9.26 h) is compromised for imaging of zinc biodistribution because its daughter isotope 62Cu is also a positron emitter that may confound the interpretation of the PET images. Nevertheless, 62Zn has been effectively used preclinically as a zinc biomarker of pancreatic exocrine function (9). 65Zn is impractical for clinical imaging because of its 244-d T1/2 but has served as a zinc biomarker in central nervous system investigations (10). The potential of 63Zn as a PET imaging tracer is supported by its attractive physical decay characteristics (T1/2, 38.5 m; mean β+ energy, 0.99 MeV; total β+ intensity, 93%) and the feasibility of cyclotron production via the 63Cu(p,n)63Zn reaction in low-energy proton accelerators (11,12). Although the shorter T1/2 of 63Zn may limit its ability to assess longer biologic turnover times, it is sufficient to monitor initial transport of zinc from the blood to tissues and further transport processes that have turnover times in the order of 2 h or less. For example, 63Zn should be well suited to study transport kinetics of zinc into the pancreas or prostate or across the blood–brain barrier.
Positron-Emitting Isotopes of Zinc
63Zn has been produced by irradiating natural copper foils with protons through the natCu(p,n)63Zn nuclear reaction that exhibits high cross section values at low proton energies (11,12). Because natural copper is composed of 69.2% 63Cu and 31.8% 65Cu, small fractions of 65Zn were produced via the 65Cu(p,n)65Zn reaction, which would preclude applications in clinical imaging. In the present study, we investigate the feasibility to produce pure 63Zn using isotopically enriched 63Cu as parent material. On the basis of our recent findings on solution targets (13), we now report use of a solution target filled with 63Cu-copper nitrate in dilute nitric acid to produce 63Zn. A methodology for rapid separation of the 63Zn product from 63Cu was also developed. Although lower quantities of 63Zn can be produced in a solution target relative to a solid target, isotopically enriched 63Cu foils are not commercially available, and the solution target approach avoids the need to dissolve the target after irradiation, thus saving time in the purification process. The purification process was automated using a radiochemistry module built in-house. Finally, the 63Zn-zinc citrate product was preliminarily evaluated for uptake characteristics by biodistribution in normal B6.SJL mice.
MATERIALS AND METHODS
The AG 50W-X8 (H+ form, 200–400 mesh) resin (Bio-Rad) was used as received and slurry-packed with water into a glass Econo-Column (Bio-Rad). The water (18 mΩ) was purified in-house (Barnstead Nanopure system by Thermo Scientific). The HCl and HNO3 were obtained as trace metal grade from Fisher Scientific. Isotopically enriched 63Cu metal (99.9% enrichment) was obtained from Cambridge Isotope Laboratories. Acetone and NaHCO3 were obtained from Sigma-Aldrich. Sterile 4% sodium citrate solution, U.S. Pharmacopeia (USP), was obtained from Fenwal Inc. Sep-Pak CM Light cartridges (Waters Corp.) were prerinsed with 5 mL of water. The radioactivity readings were recorded using a CRC dose calibrator (#480 setting, CRC-55tPET; Capintec).
Production and Purification of 63Zn
A PETtrace cyclotron (GE Healthcare) was used. The solution target was developed in-house as previously described (14). Briefly, it consisted of a 1.6-mL water-cooled tantalum target insert that was separated from the cyclotron by dual foils (aluminum and Havar [Goodfellow]) with a helium cooling channel in between. Incident proton energy was degraded from 16.4 to approximately 14 MeV at the target solution. The isotopically enriched 63Cu powder (99.9% enriched, 1.0 g, 15.9 mmol) was dissolved in excess HNO3 and then evaporated to dryness under vacuum to form 63Cu(NO3)2 ∙ xH2O. By weighing the dried material (3.8 g), we estimated that the ratio of water molecules to copper nitrate was approximately 3. To prepare a 1.7 M 63Cu(NO3)2 target solution, 0.74 g of 63Cu(NO3)2 ∙ xH2O and 12 μL of concentrated nitric acid were added to sufficient water to bring the total volume to 1.8 mL. The concentration of HNO3 (∼0.1N) was sufficient to prevent in-target precipitation of copper hydroxide while reducing water radiolysis (13).
The Cu(NO3)2 ∙ xH2O solution (1.8 mL) was loaded into the cyclotron target using an automated valve system (13,14). The target was pressurized with air (40–45 psi) and irradiated at 20 μA for 60 min. Pressure rose in the target until reaching a plateau of 110–130 psi at approximately 20 min after the start of irradiation. After irradiation, the target material was delivered to an in-house–developed automated radiosynthesis module (Fig. 1) for further processing. The target material was first delivered into a collection vial in the hot cell. The target was rinsed with water (1.8 mL) that was delivered to the same collection vial to yield a final volume of approximately 3.6 mL. Both 63Zn and 63Cu were trapped on a column of AG 50W-X8 resin (6 g), which was prewashed with 4N HNO3 (5 mL) and then flushed with water (75 mL) before each production and placed in a dose calibrator for measurement of trapped radioactivity. The 63Zn solution was loaded onto the AG 50W-X8 column at a flow rate of approximately 2 mL/min. The column was washed with 9 mL of water to remove 13N and 11C byproducts that were formed during the irradiation. Following the work of Guerra-Gómez et al. (12), the 63Zn was eluted with 0.05 N HCl in 90% acetone (30 mL) and transferred to a 100-mL neutralization flask that was prefilled with 8 mL of a 0.3 M NaHCO3 solution. After brief stirring, the resulting mixture was passed through a carboxymethyl cartridge (CM Sep-Pak Light; Waters) under 20 psi driving pressure. The carboxymethyl cartridge was washed with 0.03 M NaHCO3 (10 mL) to remove the acetone without loss of 63Zn. Finally, the product was eluted from the carboxymethyl cartridge with 2–5 mL of 4% sodium citrate USP solution, passed through a 0.2-μm sterilizing filter, and collected in a sterile empty vial. In initial runs, referred to as non-cGMP (current good manufacturing practice), metal needles were avoided, but in final runs performed according to cGMP, disposable metal needles were used in the process of sterile filtration, procurement of the quality control (QC) sample, and dilution of the product (2 mL) with sterile saline (8 mL).
Schematic of automated module for preparation of 63Zn-zinc citrate. Valves V1–V5 are composed of a disposable cassette that is mounted to front of module. All other valves are nondisposable Teflon (DuPont) solenoid valves.
The 63Cu(NO3)2 was recycled by washing the AG 50W-X8 column in retrograde direction with water (20 mL), followed by elution (again retrograde) of the 63Cu with 4N HNO3 (∼20 mL). The column was further washed with water (75 mL) to remove any residual acid. In initial runs, the AG column was reused. For the final runs that were performed according to cGMP, the AG column was prepared fresh before each run. The recovered 63Cu(NO3)2 solution was dried under vacuum and the residual 63Cu(NO3)2 ∙ xH2O reconstituted in 0.1 M HNO3 as described above.
QC of 63Zn-Zinc Citrate Product
The 63Zn-zinc citrate product was submitted for standard QC analyses for radiopharmaceutical preparation, including optical clarity by appearance, pH, radionuclidic identity (half-life and high-purity germanium γ spectroscopy, DSA-1000; Canberra), radionuclidic purity (high-purity germanium spectroscopy), residual solvents (GC-FID; SRI Instruments), filter integrity, pyrogens (endotoxin test), and sterility. For radiochemical and chemical purity, the QC samples were analyzed using a high-performance liquid chromatography (HPLC) system (Dionex ICS-5000; ThermoFisher), equipped with an IonPac CS5A analytic column (4 × 250 mm; Dionex, ThermoFisher) and PC10 postcolumn pneumatic delivery system (Dionex, ThermoFisher) to mix the dye 4-(2-pyridylazo)resorcinol (PAR) (Dionex, ThermoFisher) for spectrophotometric detection at 530 nm of Zn+2, Cu+2, Fe+3, and Ni+2. The flow rate of the mobile phase (MetPac Eluent; Dionex, ThermoFisher) was 1.2 mL/min. The flow rate of PAR diluent was 0.6 mL/min. The HPLC also had in-line radioactivity detection (Carroll and Ramsey). Because the radioactivity detector was placed before the postcolumn PAR dilution manifold, there was an approximate 0.3-min delay between the radioactivity and ultraviolet data recording.
Biodistribution and PET Imaging of 63Zn-Zinc Citrate in Mice
Biodistribution characteristics of 63Zn-zinc citrate were investigated in male B6.SJL mice (n = 4) (Jackson Laboratories) under the approval of the Mayo Clinic Institutional Animal Care and Use Committee. Animals were administered 63Zn-zinc citrate (∼1.8–3.6 MBq) into a tail vein and euthanized at 60 min for biodistribution analysis. Tissues were weighed and counted for radioactivity. Biodistribution data were expressed as standardized uptake value (SUV) = (counts/g)tissue/(injected dose counts/weight of mouse (g)). In 1 mouse, dynamic small-animal PET (Inveon; Siemens) images were acquired after intravenous injection of 14.8 MBq of 63Zn-zinc citrate. The mouse was anesthetized using 1.5% isoflurane gas. Images were reconstructed using an ordered-subset expectation maximization iterative algorithm. Image resolution was compared for 63Zn and 18F by imaging a microresolution phantom filled at a radioactivity concentration similar for the 2 PET radionuclides (∼3.7 MBq/mL).
Statistics
Biodistribution data were expressed as mean ± SD. Statistical comparison of analytic data between groups of runs was performed using the 2-tailed Student t test. Statistical comparison of intracerebral regional uptake of 63Zn was performed using ANOVA. P values of less than 0.05 were considered statistically significant.
RESULTS
Production and Isolation of 63Zn
Proton irradiations of 63Cu(NO3)2 solutions (1.7 or 1.23 M) were performed for 60 min at 20 μA (Table 2). After the target rinse dilution to approximately 0.05 M HNO3, both the 63Cu and the 63Zn were trapped on the AG 50W-X8 cation-exchange resin (Fig. 1). A further 9 mL of water rinse removed a large fraction of the 13N, 11C, and 18F contaminants generated during the irradiation. 63Zn was eluted from the AG column by washing with 30 mL of 0.05 M HCl in 90% acetone. The elution of 63Zn from the AG column was followed by monitoring the amount of radioactivity in the dose calibrator. The 63Zn was further processed by neutralization with NaHCO3, trapping on a carboxymethyl cartridge, rinsing with 10 mL of a 0.03 M NaHCO3 solution, elution with 2–5 mL of 4% sodium citrate USP solution through a 0.2-μm sterilizing filter, and collection in a sterile empty vial. The time of processing was 30–36 min. Calculated saturated yields (corrected) of processed 63Zn-zinc citrate were 309 ± 17 and 151 ± 29 MBq/μA, respectively, for 1.7 and 1.23 M 63Cu(NO3)2 solutions (Table 2). An uncorrected yield (at the end of processing) of 1.53 ± 0.10 GBq of 63Zn-zinc citrate was obtained from 60-min irradiations of a 1.7N 63Cu(NO3)2 solution (n = 3). This yield was similar to the approximate 1.4-GBq yield reported by Guerra-Gómez et al. by irradiation and processing of solid natural copper foil (12). 63Cu(NO3)2 was successfully recovered (>85% recovery) and reirradiated several times with no degradation of radionuclidic purity of 63Zn product.
Production Yield, Specific Activity, and Metal Impurities in 63Zn-Zinc Citrate Preparations
QC Tests
All standard radiopharmaceutical QC tests were passed, including appearance, half-life (38.5 ± 0.1 min), pH (6.5), endotoxins, and sterility. Representative radio–high-performance liquid chromatograms are shown in Figure 2. A single radioactive peak at 6.3 min corresponded to the nonradioactive Zn+2 retention time of 6.6 min, consistent with the time delay of 0.3 min between the radioactivity detector and the following ultraviolet detector. The analysis of metal ions in the product showed significant levels (2.6–40.5 μg/batch) of nonradioactive Zn+2, Cu+2, and Fe+3. Ni+2, however, was not detected in QC samples at a minimum detection level of approximately 1 μg/mL. Copper levels were low but significantly higher in the non-cGMP product than in the cGMP product (40 ± 16 vs. 2.7 ± 1.8 μg) likely because the non-cGMP product was prepared using recycled AG resin that had retained small quantities of raw material 63Cu that were subsequently released in later runs. In contrast, the iron levels were higher in the cGMP runs relative to the non-cGMP runs (28.2 ± 1.7 vs. 13.0 ± 2.1). The greater use of disposable stainless steel needles during cGMP processing is likely responsible for the higher levels of iron in the cGMP runs. Radionuclide purity was found to be greater than 99.9% by high-purity germanium γ spectrometry after the end of synthesis, with the known major emissions at 511 keV (185.5%), 670 keV (8.2%), and 962 keV (6.5%) (Fig. 3A). There were no detectable γ emissions above background remaining in the samples 24 h after synthesis (Fig. 3B). Specific activities of 41.2 ± 18.1 MBq/μg (uncorrected) for the 63Zn product were obtained. Thus, for a 370-MBq dose for a human study, approximately 9 μg (0.14 μmol) zinc would be administered. This level of zinc would represent a negligible amount, compared with the plasma levels of zinc of approximately 10 μM (10).
Radio–high-performance liquid chromatograms of cGMP 63Zn-zinc citrate product. (A) UV chromatogram (530 nm) for reference standards for 2.5 mg/L each of Fe+3, Cu+2, Ni+2, and Zn+2. (B) UV and radio–high-performance liquid chromatograms for representative cGMP run of 63Zn-zinc citrate product. There is 0.3-min delay between UV detector and radioactivity detector. Rad. = radio; UV = ultraviolet.
High-purity germanium detector γ spectrometry of 63Zn-zinc citrate product at 90 min (A) after end of synthesis and 24 h (B) after end of synthesis.
Biodistribution and PET Imaging in Normal Mice
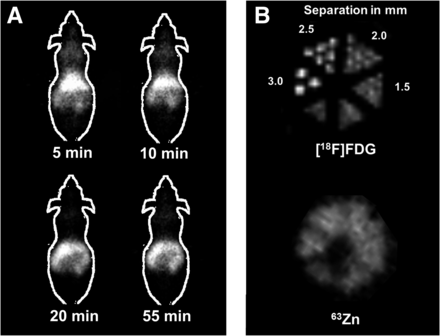
The biodistribution at 1 h showed the pancreas to have the highest SUV of 8.8 ± 3.2, with high uptake also seen in the liver, kidney, and upper intestine (Table 3). Intestinal uptake likely reflected secretion of radioactivity by the pancreas. The whole-brain SUV was 0.24 ± 0.09, showing moderate retention in the brain. The intracerebral regions did not show statistically significant differences in SUVs. Thus, brain uptake of 63Zn was similar over the regions studied. The small-animal PET images were consistent with the biodistribution (Fig. 4A), showing high uptake in abdominal tissues (liver and gastrointestinal tract).
Biodistribution of 63Zn-Zinc Citrate in Normal B6.SJL Mice (n = 4) at 1 Hour After Intravenous Administration
Small-animal PET images of normal mouse (A) after intravenous administration of 63Zn-zinc citrate, and microresolution phantom (B) scanned with solutions of either 63Zn or 18F. Distance between filled capillary tubes of each resolution group is shown in mm. 63Zn was not capable to resolve capillary tubes to maximum distance of 3.0 mm, whereas 18F allowed for resolution to 2–2.5 mm.
Microresolution Phantom Study
PET images of the microresolution phantom showed that capillary tubes filled with 63Zn solution could not be resolved for tube separations up to 3 mm, whereas 18F allowed for visualization of tube separations in the range of 2.0–2.5 mm (Fig. 4B).
DISCUSSION
Potential applications for PET imaging of zinc dynamics in a broad spectrum of diseases (1–10) motivates the development of a practical method for producing 63Zn. Of the 3 known positron-emitting zinc isotopes, 63Zn has the most favorable properties for quantitative PET imaging in humans (Table 1), although its 38.5-m half-life will limit investigations to under 2–3 h. Nevertheless, this time frame is sufficient to determine initial biodistribution patterns of zinc after intravenous injection and evaluation of fast to moderate tissue turnover times. The average positron energy of 0.992 MeV for 63Zn is significantly higher than that of 18F (0.25 MeV) but only slightly higher than 68Ga (0.84 MeV) and 124I (0.82 MeV). However, in comparison with 124I, the high-energy γ emissions from 63Zn are benign (Table 1). Thus, imaging spatial resolution is anticipated to be inferior to 18F but on par with 68Ga. Indeed, the phantom study in this work showed poorer spatial resolution in a small-animal PET resolution phantom relative to 18F and noisier small-animal PET images than typically seen with 18F-labeled radiotracers. These results are consistent with a detailed study of imaging of nonstandard PET radionuclides with small-animal PET (15).
As far as we are aware, this is the first reported method for producing radionuclidically pure 63Zn ion for use as a PET imaging probe. An earlier study by Guerra-Goméz et al. (12) produced 63Zn and small levels of 65Zn (T1/2, 244 d) contaminant after proton irradiation of natural copper foils. Because natural copper comprises 69.2% 63Cu and 30.8% 65Cu, enriched 63Cu is modestly expensive and readily available as a starting material for solution targetry. We are not aware of a commercial source of isotopically enriched 63Cu foil, but it could be electroplated onto a solid target mounting surface. Even so, our method would be more practical because it eliminates the need for acid dissolution of the solid target before isotope separation. The yields of 63Zn-zinc citrate in our study (1–1.5 GBq) are sufficient to perform several human studies with the same batch (assuming multiple PET scanners are available simultaneously). Production yields can be augmented by increasing the 63Cu-copper nitrate concentration in the target solution or increasing irradiation time. We are also working on further improvement of the target design to enhance heat transfer that we anticipate will support higher beam currents.
The final chemical form of 63Zn in our method is zinc citrate, determined by the final elution of the carboxymethyl cartridge with commercially available isotonic sodium citrate solution. 63Zn can also be eluted from the carboxymethyl cartridge using an isotonic histidine chloride solution (data not shown), but the elution efficiency is reduced. An advantage of using an isotonic 4% sodium citrate solution is that a USP-grade solution is commercially available for compliance with cGMP. We would assume that after entering the bloodstream, zinc would equilibrate among several potential binding agents, including serum albumin (16). Although albumin binds zinc with high affinity, the binding is characterized as exchangeable (17). Animal studies with 65Zn have shown rapid exchange of the blood and tissue compartments (18–20). The QC HPLC method includes measurement of zinc, iron, copper, and nickel ions in the product solution. On the basis of available literature values of LD50 (lethal dose 50: dose at which 50% of animals are killed) for intravenous administration in animals, we have placed the maximum acceptable limits of zinc, iron, copper, and nickel to be 0.026, 4.06, 1.03, and 25.8 g/L, respectively. The measured levels were well under 1% of these limits for all the metals.
The initial evaluation of 63Zn-zinc citrate biodistribution in normal mice agrees with previous results with 65Zn in rats (19). Zinc is essential for the normal processing, storage, secretion, and action of insulin in pancreatic β cells (21). The site of highest uptake of intravenously administered radiozinc is the pancreas, in which 2 Zn+2 ions coordinate 6 molecules of insulin within the storage vesicles of β cells (21). It is anticipated that 63Zn PET may play an important role for the noninvasive assessment of zinc turnover in the pancreas in metabolic diseases, including metabolic syndrome, obesity, and diabetes. The distribution into the upper intestine at 60 min is likely due to pancreatic secretion of 63Zn into the upper gut.
Liver uptake is also prominent because the liver is a highly metabolic organ that requires high levels of zinc associated with metabolic enzymes. As previously noted in other cancer types (4–6), hepatocellular carcinoma tumors consistently show marked decreases in zinc concentration, compared with normal liver (22), and thus zinc may serve as a biomarker for the early identification of malignancy. Other potential applications in the liver include monitoring of changes in liver zinc homeostasis in chronic active hepatitis and cirrhosis (23).
Finally, murine brain uptake of 63Zn was moderately low but significant. Zinc is an important cofactor in neurotransmission in γ-aminobutyric acid– (24), glutamate- (25), and glycine-mediated (26) processes. Kanayama et al. (27) showed that 65Zn uptake in rat brain at 30 min was severalfold higher than any of the other metal ions (beryllium, scandium, vanadium, chromium, manganese, iron, cobalt, arsenic, selenium, rubidium, strontium, yttrium, zirconium, technetium, and ruthenium) after administration as inorganic salts, demonstrating the robust transport of zinc across the blood–brain barrier. Interestingly, the same group showed markedly higher uptake of radioactive tracers of zinc, manganese, and rubidium in intracerebral C6 glioma tumors (28). In addition to potential 63Zn PET imaging applications in neurology and neurooncology, we anticipate imaging applications in AD and Huntington disease. In these neurodegenerative diseases, a clear association has been made for zinc and copper metal ions in amyloid-β plaque formation and stabilization (7,8). Amyloid-β protein is reversibly precipitated by zinc and copper and coordinates these metals in plaques (29). Intracerebral zinc levels are highly abnormal in AD: postmortem analysis of brain samples in patients with AD showed that cortical zinc (but not copper) levels correlate with cognitive impairment (30). Metal chelator therapy directed at altering intracerebral zinc distribution is in active clinical investigation in AD (8), whereas zinc sulfate has been proposed as a therapy in Huntington disease (31). 63Zn PET represents a new tool to begin to evaluate zinc transport in these various disease states and the effects of zinc-related therapies. Although the 38.5-min half-life of 63Zn will limit measurements of slower (>2 h) intracellular zinc turnover times, it is anticipated that dynamic PET imaging data may provide quantitative assessments of transport and more rapid turnover processes.
CONCLUSION
A production method has been developed for the first time, to our knowledge, for radionuclidically pure 63Zn based on solution target methods. The radiopharmaceutical preparation has been successfully tested according to standard cGMP QC tests. Uncorrected yields (at the end of the 30-min process) of 1.07 ± 0.22 GBq were obtained using a 1.23 M 63Cu nitrate solution and proton irradiation at 20 μA for 60 min. If greater quantities of 63Zn are needed, production levels can be readily increased by increasing the 63Cu nitrate concentration or increasing irradiation time. Specific activity is sufficient not to significantly increase zinc levels in the blood in animal and human studies. A practical and efficient method of production of 63Zn-zinc citrate has been developed that will enable PET imaging studies of zinc biodisposition and kinetics in animal and human studies.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by DOE (TRD/APB) DE-SC0008947. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Ray Steichen and Teresa Decklever at Mayo Clinic for their helpful assistance.
Footnotes
Published online Jul. 21, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication April 8, 2014.
- Accepted for publication May 30, 2014.