Abstract
The development of a PET radioligand selective for I2-imidazoline binding sites (I2BS) would enable, for the first time, specific, measurable in vivo imaging of this target protein, along with assessment of alterations in expression patterns of this protein in disease pathophysiology. Methods: BU99008 was identified as the most promising I2BS radioligand candidate and radiolabeled with 11C via methylation. The in vivo binding properties of 11C-BU99008 were assessed in rhesus monkeys to determine brain penetration, brain distribution, binding specificity and selectivity (via the use of the unlabeled blockers), and the most appropriate kinetic model for analyzing data generated with this PET radioligand. Results: 11C-BU99008 was demonstrated to readily enter the brain, resulting in a heterogeneous distribution (globus pallidus > cortical regions > cerebellum) consistent with the reported regional I2BS densities as determined by human tissue section autoradiography and preclinical in vivo PET studies in the pig. In vivo competition studies revealed that 11C-BU99008 displayed reversible kinetics specific for the I2BS. The multilinear analysis (MA1) model was the most appropriate analysis method for this PET radioligand in this species. The selective I2BS blocker BU224 was shown to cause a saturable, dose-dependent decrease in 11C-BU99008 binding in all regions of the brain assessed, further demonstrating the heterogeneous distribution of I2BS protein in the rhesus brain and binding specificity for this radioligand. Conclusion: These data demonstrate that 11C-BU99008 represents a specific and selective PET radioligand for imaging and quantifying the I2BS, in vivo, in the rhesus monkey. Further work is under way to translate the use of 11C-BU99008 to the clinic.
The ability of the α2-adrenoceptor agonist clonidine and the antagonist idazoxan to label a subpopulation of binding sites, not displaceable by the endogenous ligand noradrenaline, led to the discovery of the imidazoline binding sites some 20 y ago. These binding sites have subsequently been divided into 3 groups: the imidazoline1 binding site, which is preferentially labeled by 3H-clonidine; the imidazoline2 binding site (I2BS), which is preferentially labeled by 3H-idazoxan; and the imidazoline3 binding site, which is an atypical imidazoline site found on pancreatic β-cells (1).
I2BS are known to reside on the mitochondrial membranes of astrocytes (2). Changes in postmortem binding density of the I2BS have implicated them in a range of psychiatric conditions such as depression and addiction, along with neurodegenerative disorders such as Alzheimer disease and Huntington chorea (3). Functional interactions in preclinical models have also been shown in relation to the opioid system, in which I2BS ligands have been shown to affect tolerance to morphine (4) and alleviate some of the morphine withdrawal syndrome in rats (5). I2BS ligands have also been shown acutely to affect feeding and appetite by an as-yet undetermined mechanism (6). The location of I2BS on glial cells and the possibility that they may in some way regulate glial fibrillary acidic protein (7) have led to increased interest into the role of I2BS and I2BS ligands in conditions characterized by marked gliosis. The density of I2BS has been shown to increase in Alzheimer disease postmortem (3), and it has also been suggested that I2BS may be a marker for human glioblastomas (8). Subsequent publications added weight to this argument, showing that the density of I2BS is increased in vivo with heat-induced gliosis (9). Additionally, Callado et al. have shown not only an increase in the I2BS in human gliomas but also that this increase in binding sites was correlated with the severity and malignancy of the glioma (10).
PET is an in vivo imaging technique that uses radioligands as selective molecular probes to map the location and density of specific proteins. The development of a selective I2BS PET radioligand would allow for the characterization of I2BS in vivo and its regulation in disease states. Several ligands selective for I2BS have been reported, but only 2 potential PET radioligands have been reported to date: the radiosynthesis of 11C-benazoline, but its study in vivo has not been reported (11), and the radiosynthesis and in vivo imaging evaluation of 11C-2-(3-fluoro-4-11C-tolyl)-4,5-dihydro-1H-imidazole in nonhuman primates (12), although the specific binding signal appears to be low for this radioligand.
We have recently reported the synthesis, in vitro and in vivo evaluation, and radiosynthesis of a PET radioligand for the I2BS, 11C-BU99008 (13,14). In this article, we report the preclinical in vivo evaluation of 11C-BU99008, for imaging I2BS, in the rhesus monkey brain.
MATERIALS AND METHODS
Chemicals
3H-BU99008 (specific activity, 1.04 TBq/mmol) was custom synthesized by Sibtech. Challenge drugs moclobemide and lazabemide were obtained from commercial suppliers: Sigma-Aldrich Co. Ltd. and Tocris Biosciences. Dr. Stephen Husbands synthesized the BU224. All other chemicals and reagents were purchased from commercial suppliers and used without further purification.
Animals
All animal experiments were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986. PET imaging experiments in rhesus monkeys were conducted in accordance with a protocol approved by the Yale University Institutional Animal Care and Use Committee.
In Vitro Competition Binding Studies
Membrane preparation, competition binding studies, and data analysis were conducted as previously described (13) with the following alterations. Rat (male; Wistar; weight, 250–300 g) and Cynomolgus monkey brains were used and resulting membrane preparations stored at −80°C. The displacement binding studies for the 3H-BU99008 (1 nM) were conducted at 37°C in assay buffer (50 mM Tris-HCl, 140 mM NaCl, 1.5 mM MgCl2, 5 mM KCl, 1.5 mM CaCl2, pH7.4), and radioactivity was determined using a Tricarb 2900 β-counter (PerkinElmer). Protein content was determined using a Pierce bicinchoninic acid kit.
PET Imaging Studies in Rhesus Monkeys (Macaca mulatta)
Radiochemistry
11C-BU99008 was prepared by N-alkylation of the desmethyl precursor BU99007 with 11C-CH3I in the AutoLoop synthesis module (Bioscan). A description of the synthetic methods can be found in the supplemental information (Supplemental Fig. 1; available at http://jnm.snmjournals.org).
Study Design
Two Rhesus monkeys (female; weight, ∼6 and ∼7 kg; age, 7 and 8 y) were used, with scanning days at least 14 d apart. Each animal had 5 scanning days. Each scanning day consisted of a baseline scan with 11C-BU99008 (120 min); after this, animals received an intravenous injection of blocking drug over a 10-min period, approximately 10 min before initiation of a second scan with 11C-BU99008 (120 min), to determine binding specificity and selectivity of the radioligand (Supplemental Table 1). The administration of the specific I2BS ligand, BU224, was 0.01, 0.03, and 0.3 mg/kg for monkey 1 and 0.01, 0.03, and 0.1 mg/kg for monkey 2. To assess the selectivity of binding to I2BS, both animals received an injection of the reversible monoamine oxidase A (MAOA) inhibitor moclobemide (1 mg/kg) and the reversible MAOB inhibitor lazabemide (0.5 mg/kg). Data acquisition started simultaneously with ligand injection. Vital signs were monitored at least 4 times per hour and more frequently after injection of tracer and blocking drugs.
MR Imaging
Images were acquired for each monkey on a 3.0-T Trio scanner (Siemens), using an extremity coil. T1-weighted images were acquired in the coronal plane with a spin-echo sequence (echo time, 3.34; repetition time, 2,530; flip angle, 7°; section thickness, 0.50 mm; field of view, 140 mm; image matrix, 256 × 256 × 176 pixels; matrix size, 0.547 × 0.547 × 0.500 mm). The whole-brain image was cropped to 176 × 176 × 176 pixels using MEDx software (Medical Numerics) before coregistration with PET image data.
PET Imaging Procedures
Animals were sedated with an intramuscular injection of ketamine hydrochloride (10 ± 2 mg/kg), approximately 2 h before the start of scanning, transported to the PET facility, anesthetized using isoflurane, intubated, and maintained on oxygen and 1.5%–2.5% isoflurane throughout the study. PET scans were obtained on the Focus 220 PET scanner (Siemens Preclinical Solutions), with a reconstructed image resolution of approximately 1.5 mm. After a transmission scan, 170 ± 14 MBq (4.6 ± 0.4 mCi; mass dose, 0.08 ± 0.02 μg/kg) of 11C-BU99008 was injected over 3 min. List-mode data were acquired for 120 min and binned into sinograms with the following frame timing: 6 × 30 s, 3 × 1 min, 2 × 2 min, and 22 × 5 min. Dynamic scan data were reconstructed with a filtered-backprojection algorithm with corrections for attenuation, normalization, scatter, and randoms.
Arterial Blood Sampling
Arterial blood samples were collected for the determination of whole blood and plasma input functions and metabolite analysis and plasma-free fraction of 11C-BU99008. These procedures are described in detail in the supplemental information.
Regional Time–Activity Curve Computation
An existing region-of-interest (ROI) map defined on a template brain (a representative MR image of a rhesus brain) was used. The following, a priori defined, ROIs were examined: cingulate, frontal, insula, and occipital cortex; brain stem; pons; cerebellum; caudate; putamen; globus pallidus; and thalamus. A nonlinear transformation was estimated using the Bioimagesuite software (http://www.bioimagesuite.org/) to transfer the ROI template to the MR image of each animal used during this study. These regions were then transferred to the PET images based on a rigid transformation matrix (15) and used to generate time–radioactivity curves (time–activity curve).
Kinetic Modeling
Regional time–activity curves were analyzed using 1- and 2-tissue-compartment models (1TC and 2TC) and multilinear analysis (MA1) (16) to calculate regional distribution volume (VT). MA1 is a linear method related to Logan analysis but with less noise-induced bias. Like Logan analysis, data are fitted starting at a specified time, t*; here t* is 20 min. The optimal model was based on quality of fit and the uncertainty (SE) of the VT parameter estimate. For blocking studies, because there was no suitable reference region, a graphical method was used to calculate the nondisplaceable volume of distribution (VND) and global receptor occupancy (17).
To derive the blocking dose needed to induce 50% global and regional receptor occupancy (ED50), the 3-parameter dose–response curve was used (GraphPad Prism, version 6.0 d for Mac OS X; GraphPad Software [www.graphpad.com]). For the global ED50, the occupancy calculated from Cunningham et al. (17), which accounts for VND, was used. For regional ED50, the percentage reduction in VT values from baseline after various blocking doses of drug, which does not account for VND, were calculated and fitted to this equation.
RESULTS
In Vitro Competition Binding Studies
3H-BU99008 in vitro competition data demonstrated a 2-site fit to the rodent brain with BU224, exhibiting a half maximal inhibitory concentration for the high-affinity site value of 50.5 ± 12.9 nM (Table 1), consistent with previous data (13). In contrast, competition of BU224 in cynomolgus brain yielded a single-site fit, with a half maximal inhibitory concentration value of 130.2 ± 33.9 nM (Table 1). The competition of 3H-BU99008 from both rat and cynomolgus brains by the MAOB inhibitor lazabemide exhibited poor inhibition of binding, and the MAOA inhibitor moclobemide showed no inhibition at the highest concentration used (Table 1).
In Vitro I2BS Binding Affinities of Blocking Drugs in Rat and Nonhuman Primate Brain Using 3H-BU99008
Radiochemistry
Injection-ready 11C-BU99008 was successfully synthesized with a chemical yield of 32% ± 17% (decay-corrected), radiochemical purity of greater than 99%, and a specific activity of 146 ± 33 MBq/nmol (3.95 ± 0.90 mCi/nmol, n = 19) at the end of synthesis. The identity of the radiolabeled product was confirmed by coinjection with a sample of authentic BU99008, which, under the same elution conditions, showed an identical retention time.
In Vivo Blood Data
Free fraction of 11C-BU99008 in the plasma was high, at 0.68 ± 0.07 (n = 19). The amount of total radioactivity measured in plasma was similar between baseline scans and after administration of either the MAO inhibitors or the I2BS inhibitor (Fig. 1A). In addition, radio–high-performance liquid chromatography analysis revealed 11C-BU99008, under baseline conditions, to be rapidly metabolized in plasma, with the parent compound representing about 50% of the total radioactivity 20 min after administration (Fig. 1B). However, there was a small decrease in the parent fraction of 11C-BU99008 after administration of all 3 inhibitors, compared with data acquired under baseline conditions, for which the parent compound represented about 50% of the total radioactivity at 15 min after administration (Fig. 1B).
Mean total plasma radioactivity (A) and metabolite data (B) for 11C-BU99008 scan. ● = baseline scan data; △ = blocking scan data using MAO inhibitors; □ = blocking scan data using BU224. Each point represents mean of all scans in that group; vertical bars represent SD.
In Vivo PET Studies
Representative baseline PET images and corresponding time–activity curves for 11C-BU99008 uptake into the rhesus brain are given in Figures 2B and 3A, respectively. 11C-BU99008 readily entered the brain, with the highest uptake observed in the globus pallidus, caudate, and thalamus; with moderate uptake in the cortical and putamen regions; and with lowest uptake in the cerebellum and occipital cortex. Peak radioactivity concentrations were observed approximately 15–25 min after administration of 11C-BU99008, followed by a slow washout from all regions (Fig. 3A).
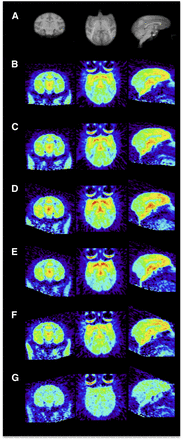
Representative coronal, transverse, and sagittal images of 11C-BU99008 uptake in rhesus brain. Images are summed from 30 to 45 min after radioligand injection and displayed as SUVs. (A) Structural MR imaging. (B and C) Baseline (B) and moclobemide preblock (C) (1 mg/kg). (D and E) Baseline (D) and lazabemide preblock (E) (0.5 mg/kg). (F and G) Baseline (F) and BU224 preblock (G) (0.3 mg/kg). Paired scans B and C, D and E, and F and G were obtained on same day.
Representative time–activity curves for 11C-BU99008 in selected ROIs in rhesus brain. (A) Baseline 11C-BU99008 scan. (B) 11C-BU99008 scan after administration of BU224 (0.3 mg/kg). ▪ = caudate; □ = cerebellum; △ = frontal cortex; ▼ = globus pallidus; ♢ = occipital cortex; ○ = putamen; + = thalamus.
The regional time–activity curves were analyzed by the reversible 1TC and 2TC models and by the MA1 model (16). The 2TC model produced good fits to the data, but more than 20% of fits to baseline data had unreliable VT estimates—that is, a percentage SE (%SE) greater than 20%. Poor reliability occurred most often in smaller, noisier regions and in baseline scans or scans with little effective blockade (see below). The 1TC model showed clear lack of fit in most cases. Also, the VT values from the 1TC underestimated those from the 2TC by 10%–40% (excluding 2TC values with high %SE), with the relationship of VT(1T) = 0.87 × VT(2T) – 4.2, r2 = 0.92. The MA1 method produced good fits and stable estimates for VT, with small differences in VT values using different t* values from 20 to 40 min. In the cases in which 2TC values had a %SE less than 20%, the relationship between MA1 and 2TC values was VT(MA1) = 0.94 × VT(2T) – 1.3, r2 = 0.97. On the basis of the bias from 1TC fits, and the numerous cases of high %SE from 2TC, MA1 with a t* of 20 min was chosen for derivation analysis.
Table 2 lists MA1-derived regional VT values from individual baseline scans. The baseline data acquired from the 2 rhesus monkeys were demonstrated to be reproducible and consistent throughout the course of the study (Fig. 4). When the MA1 model with a t* of 20 min was used, baseline VT values were highest in the globus pallidus (114.2 ± 24.0 mL/cm), caudate (109.7 ± 13.7 mL/cm), and thalamus (96.3 ± 8.1 mL/cm) and lowest in the cerebellum (48.1 ± 4.8 mL/cm). This rank order of regional VT for 11C-BU99008 from the baseline data (globus pallidus > cortex > cerebellum, Table 2) is consistent with reported I2BS densities and distribution determined by tissue-section autoradiography in humans (18) and in vivo pig PET (14). Furthermore, the mean rhesus VT values were significantly correlated (r2 = 0.72; P < 0.05) with the mean VT values from our previous in vivo porcine 11C-BU99008 PET data (inset, Fig. 4) ((14); S. Kealey, E.M. Turner, S.M. Husbands, et al., unpublished data, 2013, from the porcine study).
Baseline Regional VT Values (mL/cm) for 11C-BU99008 Using MA1
Bar chart showing regional distribution volume (VT) of 11C-BU99008 from 2 animals (monkey 2, open bars, and monkey 1, filled bars). These represent mean ± SD from 4 separate baseline scans. Insert shows significant correlation (r2 = 0.72; P < 0.05) between mean VT from this study (both animals) and porcine study (n = 3) ((14); S. Kealey, E.M. Turner, S.M. Husbands, et al., unpublished data, 2013, data from porcine study). Regional VT values were generated using MA1 model (t* = 20 min) for rhesus data and 1TC model for porcine data.
In vivo blocking studies using the MAOA and MAOB inhibitors moclobemide and lazabemide, respectively, did not cause any significant change in binding signal of 11C-BU99008 to any regions studied (Figs. 2C and 2E; Supplemental Fig. 2). In vivo competition using 11C-BU99008 plus increasing doses of the selective I2BS blocker, BU224, yielded a dose-dependent decrease in uptake of 11C-BU99008 in all regions studied. The highest dose administered (0.3 mg/kg) yielded an apparent near-to-full blockade, suggesting high selectivity of this radioligand for the I2BS (Figs. 2G, 3B, and 5; Table 3). The presence of a dose-dependent decrease in binding in the cerebellum suggests this is not a suitable reference region for analysis of 11C-BU99008.
Bar chart showing regional distribution volume (VT) of 11C-BU99008 and effect of increasing doses of I2BS ligand BU224: 0.01 mg/kg (striped), 0.03 mg/kg (checkered), 0.1 mg/kg (clear), 0.3 mg/kg (gray), and baseline (black). Bars represent mean ± SD; VT generated using MA1 model.
Calculated Fraction of I2BS Occupied by BU224 Using 11C-BU99008
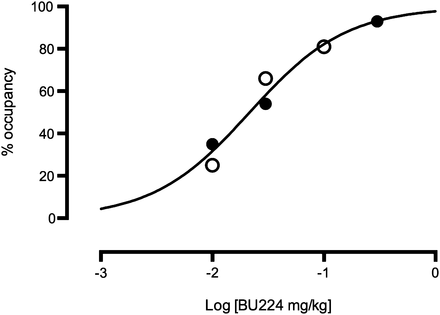
A global receptor occupancy measure was calculated for the BU224 studies using the occupancy plot (17). Occupancy values ranged from 25% to 35% for the lowest dose of BU224 (0.01 mg/kg) to 93% for the highest dose (0.3 mg/kg). These occupancies were plotted versus administered dose (d) in Figure 6, with a fit to the equation Occ = d/(d + ED50), which yielded an ED50 estimate of 0.022 mg/kg for the whole brain (Table 3).
Dose-dependent occupancy by BU224 in whole brain; occupancy calculated using occupancy plot (ED50 = 0.022 mg/kg) (17). VT data generated using MA1 model. ● = monkey 1; ○ = monkey 2.
Regional ED50 values for BU224 were also calculated for the globus pallidus, frontal cortex, and cerebellum using the percentage reduction in VT instead of the estimated global occupancy value (Supplemental Figs. 3A–3C). Calculated regional ED50 values were 0.017 mg/kg for the globus pallidus, 0.017 mg/kg for the frontal cortex, and 0.016 mg/kg for the cerebellum (Supplemental Fig. 3; Table 3).
DISCUSSION
This paper describes the radiosynthesis of 11C-BU99008 and its characterization as a novel PET radioligand for the quantification of central I2BS in vivo in rhesus monkeys.
BU99008 was selected as the most suitable compound for radiolabeling with a PET radioisotope for imaging the I2BS, as reported previously by our group (13). In addition, BU99008 exhibited selectivity and nanomolar affinity for the I2BS in the rodent and cynomolgus brain (Table 1). Interestingly, a 2-site model fit was preferred for the rodent and a 1-site fit for the cynomolgus brain tissue, suggesting that, in the rodent brain, BU99008 exhibits a degree of affinity for a second binding site, which may be unrelated to the I2BS, possibly reflecting a MAO binding component (19). Importantly, because of the nature of the I2BS being colocalized with MAOs on the outer membrane mitochondrial enzymes (20), it was essential to investigate the relative affinities of selective MAOA and MAOB inhibitors for the I2BS to proceed. In vitro studies using cynomolgus brain demonstrated MAO inhibitors to possess a low affinity for the I2BS in (Table 1). However, in the rodent brain tissue, the MAOB inhibitor possessed a similar affinity for the low-affinity binding site exhibited previously in the rodent brain by BU224 (Table 1). These data confirm the notion that the high-affinity binding component, in vitro, in both the rat and the cynomolgus brain is expected to represent the I2BS.
In view of these in vitro data, and combined with a successful radiolabeling feasibility assessment, we progressed the development of BU99008 as a PET ligand via evaluation in porcine brain, in vivo (14). In that study, 11C-BU99008 demonstrated reversible kinetics and a brain distribution consistent with the known binding site densities and localization for the I2BS protein and a dose-dependent decrease in VT after administration of the selective I2BS blocker BU224. Further studies in pigs showed a small, but relevant, binding component associated with MAO (R.J. Tyacke, S. Kealey, J. Myers, et al., unpublished data, 2013). Therefore, given that relative expression levels of I2BS and MAO differs from species to species, to progress 11C-BU99008 for use in human studies we decided to assess this PET ligand further, preclinically, in rhesus monkeys, because we predict human brain uptake and in vivo binding characteristics of this particular PET ligand would be more similar to rhesus brain than porcine brain. BU99008 was successfully radiolabeled with 11C with good reproducibility, radiochemical yields, and high specific activities.
After injection into a rhesus monkey, the radioligand 11C-BU99008 metabolized fairly quickly, with only approximately 30% of the parent compound remaining at 30 min after injection. A slight acceleration in metabolism was also observed when blocking agents were given before 11C-BU99008 injection, but this effect was modest and unlikely to affect the usability of this ligand.
In monkey brain, 11C-BU99008 displayed differential regional uptake. This heterogeneous distribution of 11C-BU99008 exhibited the following rank order: globus pallidus and other basal ganglia regions > cortex > cerebellum, consistent with the known I2BS densities and results from human tissue–section autoradiography (18) and porcine PET imaging experiments (14). Although the cerebellum showed the lowest brain uptake, there was still a decrease in VT values after BU224 blockade (Fig. 4), indicating the cerebellum would be unsuitable as a reference region. This was consistent with the findings of our previous study in pigs (14).
An assessment of the intrasubject variability was performed that yielded low to moderate variability in the VT values obtained for each ROI studied for each subject across multiple baseline scans (Fig. 4). Furthermore, comparison of interspecies variability between the rhesus monkeys used in this study and pigs used previously by our group with this PET radioligand (14) demonstrated a significant correlation (inset, Fig. 4), suggesting a degree of correspondence between these 2 species for the I2BS. 11C-BU99008, however, appears to be affected by MAO inhibitors in the pig, which is a phenomenon not exhibited in the rhesus (R.J. Tyacke, S. Kealey, J. Myers, et al., unpublished data, 2013).
The effects of the MAOA inhibitor moclobemide and the MAOB inhibitor lazabemide on 11C-BU99008 binding were determined in vivo, in rhesus monkeys, and found to cause no significant decrease in VT in any of the ROIs assessed (Supplemental Fig. 2). This key finding suggests that in rhesus monkey brain any contribution of the 11C-BU99008 signal due to binding to MAO is small or negligible and would not be expected to cause any significant interference with the assessment of I2BS binding signal in this species.
After administration of increasing blocking doses of BU224, a dose-dependent decrease in 11C-BU99008 VT was observed in all regions of the rhesus brain (Fig. 5), confirming the absence of a reference region for this PET radioligand and remaining consistent with the known distribution profile for I2BS in the brain. The dose-dependent decrease in VT observed for all ROIs studied is not thought to represent a global change unrelated to the specific binding of 11C-BU99008 and the blocking by BU224 given that a heterogeneous signal was observed across the ROI under baseline conditions, differential levels of decrease in VT values were observed for each region after increasing doses of BU224, and a plateau at a VT value of approximately 20 mL/cm was achieved for all ROIs after the highest dose of BU224 administered (0.3 mg/kg; Fig. 5). Additionally, the dose-dependent blockade by the selective I2BS inhibitor in the rhesus brain confirmed the specificity of 11C-BU99008 for the I2BS and demonstrated a blockade of approximately 90% across all ROIs at the highest dose administered (0.3 mg/kg). Interestingly, the in vivo ED50 of BU224 generated across all brain regions was consistent with the presence of 1 binding site and generated a value of 0.022 mg/kg (Fig. 6), which is consistent with the known in vitro and ex vivo properties of this compound (5).
Given these data, we predict that 11C-BU99008 should demonstrate a binding distribution profile in the human brain similar to that observed from this study in the rhesus monkey, in which the rank order of regional brain uptake for this ligand would be expected to be globus pallidus > cortical regions > cerebellum. For modeling purposes, because MA1 consistently produced good fits to the data along with reliable and stable estimates of VT for all regions of the rhesus brain studied, the use of this particular model for analysis of PET data generated using 11C-BU99008 should be considered in future studies. Work is under way to assess the utility of 11C-BU99008 as a PET ligand for in vivo imaging and quantification of I2BS in the human population, and if deemed useful, its applicability for determining alterations in I2BS density and distribution in known disease states will be investigated.
CONCLUSION
This article reports the radiolabeling and pharmacologic investigation of 11C-BU99008 as a novel I2BS PET radioligand in rhesus monkeys. In vivo distribution of 11C-BU99008 in the rhesus monkey brain demonstrated the following rank order of regional uptake: globus pallidus > cortex > cerebellum, consistent with the known distribution profile of the I2BS. 11C-BU99008 displayed reversible kinetics and specificity for the I2BS, with the MA1 model representing the most appropriate analysis method for the derivation of binding parameters for this PET radioligand. The data reported here provide evidence for 11C-BU99008 to represent a potentially useful PET imaging tool for probing the I2BS. Work is under way to progress 11C-BU99008 for assessment of its clinical utility as a PET radioligand for I2BS.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was funded by the MRC (G0801501) and GSK. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Roger Gunn and Eugenii Rabiner for interesting discussions and their continued support of this study.
Footnotes
↵* Contributed equally to this work.
Published online Apr. 7, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication September 5, 2013.
- Accepted for publication January 24, 2014.