Abstract
2135
Objectives To compare conventional hydroxyethyl starch augmented gravity sedimentation/centrifugation, with an automated microfluidic chip for leukocyte (WBC) isolation in preparation for radiolabeling.
Methods 25 pts. referred for 111InWBC imaging were prospectively investigated. 60 mL blood was withdrawn & divided into 40mL (Group 1) & 20 mL (Group 2) aliquots. Group 1 WBCs underwent conventional isolation. Group 2 WBCs were isolated with an automated microfluidic chip process using size based sorting where whole blood is displaced through a microfluidic chip by a syringe pump. Within the chip, the larger WBCs are isolated from smaller RBCs & platelets, & collected for labeling. Isolated WBCs in both groups were labeled with 27.8 MBq 111In-oxine using standard methods. Labeling efficiency, cell viability & label stability were determined. An automated cell analyzer was used to determine WBC, RBC & platelet retention in the final InWBC preparation.
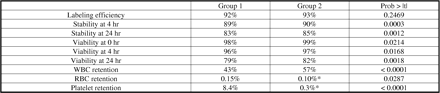
Results Labeling efficiency was similar in Groups 1 & 2. InWBC stability, viability & retention were significantly higher & RBC & platelet retention significantly lower in Group 2 (table).
Conclusions These data indicate that the microfluidic chip method provides superior WBC isolation & retention & less RBC & platelet contamination. Microfluidically isolated WBC quality was comparable or superior to that of conventionally isolated WBC’s in terms of labeling efficiency, viability, & label stability. In addition to advantages of automation & decreased personnel contact with blood, reduced RBC & platelet concentrations provided by the microfluidic process potentially could improve image quality/test accuracy.
Research Support This work was supported in part by National Institute Of Biomedical Imaging and Bioengineering Award P41EB002503 to BioMeMs Resource Center at Massachusetts General Hospital
Comparison performed using two tailed paired t- test (α = 0.05; p values < 0.05 denote statistical difference in the methods)