Abstract
Increased expression of the sodium iodide symporter (NIS) is required for effective radioiodine treatment and reporter gene imaging of breast cancer. We investigated the effect of retinoic acid on adenovirus-mediated expression of the human NIS gene in the MCF-7 breast cancer cell line. Methods: The MCF-7 cell line was infected with recombinant adenovirus carrying the human NIS gene (Rad-NIS). Levels of NIS messenger RNA (mRNA) and protein expression and radioiodine (125I) uptake were measured to evaluate adenovirus-mediated NIS gene expression in wild-type and Rad-NIS–infected MCF-7 cells after treatment with all-trans-retinoic acid (ATRA; 10−8–10−6 mol/L). Results: The transduction efficiency of adenovirus in MCF-7 cells at a multiplicity of infection (MOI) of 50 was >60%. After incubation with 10−6 mol/L ATRA, the mRNA level in Rad-NIS–infected MCF-7 cells increased to 118.5 times that of wild-type MCF-7 cells, whereas the mRNA level in wild-type MCF-7 cells showed only a 2.1-fold increase. Western blot, immunocytochemical staining, and flow cytometry analyses showed that NIS protein expression in MCF-7 cells infected with Rad-NIS increased after ATRA treatment. With ATRA treatment, the amount of 125I uptake increased in a dose-dependent manner (P < 0.001). The 125I uptake in wild-type MCF-7 cells increased 3.1-, 5.5-, and 7.6-fold with treatment with 10−8, 10−7, and 10−6 mol/L ATRA, respectively. Rad-NIS–infected cells showed a 4.0-fold increase in 125I uptake. Treatment of Rad-NIS–infected cells with 10−8, 10−7, and 10−6 mol/L ATRA increased 125I uptake by 4.9-, 8.2-, and 27.6-fold, respectively, compared with wild-type MCF-7 cells. The level of NIS expression in Rad-NIS–infected MCF-7 cells treated with 10−6 mol/L ATRA (245.0 ± 13.7 pmol/106 cells) was much greater than the sum of the expression levels seen in ATRA-treated wild-type cells and Rad-NIS–infected wild-type cells. Conclusion: Retinoic acid increases adenovirus-mediated NIS expression in MCF-7 cells. Our results indicate that improved efficiency of NIS gene therapy or reporter imaging in breast cancer may be possible with retinoic acid treatment.
The active transport of iodine into the thyroid gland is the first step in the synthesis of thyroid hormones and is mediated by the sodium iodide symporter (NIS), which is a transmembrane glycoprotein on the basolateral membrane of the thyroid follicular cells (1). Thyroidal accumulation of iodine allows the use of radioiodine for the diagnosis and postoperative treatment of differentiated thyroid cancer. Breast cancer is the most common cancer in women; yet no curative therapy exists for recurrent and metastatic breast cancer. Lactating breast tissue and some breast cancers have been shown to express NIS and thereby accumulate iodide and pertechnetate (2,3). Therefore, increased NIS expression in breast cancer cells may allow radioiodine techniques to be used in the diagnosis and treatment of breast cancer. However, the expression level of NIS in breast cancer cells is variable and, even in NIS-expressing tissues, iodide uptake is not sufficient for clinical routine diagnostic imaging or treatment (4). The potential use of radioiodine in breast cancer will require the levels of NIS expression to be increased.
Induction of the NIS gene and increased iodide uptake have been demonstrated in a transfected MCF-7 breast cancer cell line (5) and breast cancer cells transduced with an adenovirus carrying a human cytomegalovirus (CMV) promoter-driven NIS gene (6,7). Recently, treatment with retinoic acid, either alone or in combination with dexamethasone, has been shown to induce NIS gene expression and iodide uptake in estrogen receptor–positive MCF-7 cells and in an animal model with MCF-7 cells (8–13). A recent study has demonstrated that the transcription factor Nkx-2.5 is a crucial component in the transcription regulation in the mammary gland and MCF-7 cells (13). Retinoic acid is a biologically active metabolite of vitamin A and has regulatory effects on cell differentiation, proliferation, and apoptosis (14). In thyroid cancers, retinoic acid is known to induce cellular differentiation and NIS expression (15,16).
Replication-deficient adenoviruses are efficient vehicles for delivering genes into mammalian cells and are widely used for cancer gene therapy and reporter gene imaging (17). The CMV promoter, which is often present in adenoviruses, contains cis-acting DNA-binding sites referred to as retinoic acid response elements (RAREs), which are responsive to nuclear retinoic acid receptors (18,19). Retinoic acid has been shown to increase expression of adenovirus-mediated transgene expression both in vivo and in vitro (20). The presence of nuclear retinoic acid receptors in MCF-7 cells (21) and of the RAREs in CMV promoters (19) may allow the use of retinoic acid to increase the level of adenovirus-mediated NIS gene expression in MCF-7 cells. As a result, improved efficiency of NIS gene therapy and reporter gene imaging may be achieved. In this study, we investigated the effect of all-trans-retinoic acid (ATRA) treatment on adenovirus-mediated expression of human NIS in the MCF-7 breast cancer cell line.
MATERIALS AND METHODS
Cell Lines and Recombinant Adenovirus
MCF-7 human breast cancer cells were obtained from the American Type Culture Collection (lot number: 3448190) and maintained in Dulbecco's modified Eagle medium ([DMEM] Gibco-BRL) supplemented with 10% fetal bovine serum, l-glutamine (2 mmol/L), penicillin (10 IU/mL), and streptomycin (50 μg/mL), at 37°C and with 5% CO2. An E1- to E3-deleted replication-defective recombinant adenovirus carrying the human NIS gene under the control of the CMV promoter (Rad-NIS) was kindly donated by Dr. Sissy M. Jhiang (Ohio State University). A recombinant adenovirus carrying a CMV promoter-driven galactosidase gene (Rad-LacZ) has been described previously (22) and was used to evaluate the efficiency of adenovirus-mediated gene transfer. Human adenovirus 5-transformed human embryonic kidney 293 cells were maintained in DMEM supplemented with 10% fetal bovine serum, l-glutamine (2 mmol/L), penicillin (100 IU/mL), and streptomycin (50 μg/mL). The adenoviruses were plaque-purified, propagated, and titered in 293 cells using a standard method involving a CsCl gradient purification as described previously (23). The virus bands were recovered and desalted by dialysis in Tris buffer twice and a final dialysis in Tris buffer containing 5% sucrose.
Efficiency of Gene Transfer
To test the efficiency of adenovirus-mediated gene transfer in MCF-7 cells by recombinant adenovirus, cells were infected with Rad-LacZ at a multiplicity of infection (MOI) of 5 or 50 and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Two days after infection with Rad-LacZ, the MCF-7 cells were processed with fixing solution (1.0% formaldehyde and 0.2% glutaraldehyde in deionized H2O) for 5–10 min and washed twice with phosphate-buffered saline twice. The cells were then incubated with staining solution (0.4 mg/mL X-Gal, 4 mmol/L K4Fe, 4 mmol/L K4Fe, 2 mmol/L MgCI2 in phosphate-buffered saline, pH 7.2) for 4–24 h at 37°C.
ATRA Treatment and Cell Growth Inhibition
ATRA was purchased from Sigma. Stock solutions were prepared in dimethyl sulfoxide stored at temperatures below −70°C. Further dilutions were made in DMEM before use.
MCF-7 cells were plated in 6-mm dishes (1 × 104 cells per dish) and grown. Twenty-four hours after plating, cells were incubated with 10−8, 10−7, or 10−6 mol/L ATRA for 48 h. After cells were detached by brief exposure to 0.25% trypsin to give a single-cell suspension, the number of cells in each treatment group was measured using the trypan blue exclusion method. Unless otherwise noted, we performed 3 independent experiments in triplicate for all analyses.
NIS Gene Transfer and Evaluation of Expression
MCF-7 cells were plated and grown for 24 h. The cells were then incubated in the presence of varying concentrations of ATRA (0, 10−8, 10−7, or 10−6 mol/L). After a 24-h incubation with ATRA, the cells were infected with Rad-NIS at 50 MOI. The medium was replaced daily (24), and 48 h after infection NIS expression was assessed as described. Control MCF-7 cells were not infected with Rad-NIS, and in these cells NIS expression and radioiodine uptake were evaluated after incubation with ATRA for 72 h.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed to evaluate messenger RNA (mRNA) expression as described previously (25). Total RNA was isolated using Trizol reagent (Invitrogen Inc.) according to the manufacturer's protocol. The PCR primer and TaqMan probes for the mRNA of human NIS were designed using computer software (Primer Express; ABI Perkin Elmer). The sense and antisense primer sequences were 5′-GTA CCT GGA GAT GCG CTT CAG-3′ and 5′-TCG TGG CTA CAA TGT ACT GCA AA-3′, and the specific probe sequence was 5′-CAG TGC GGC TCT GCG GGA-3′. The TaqMan probe was labeled with a reporter fluorescent dye, FAM (6-carboxyfluorescein), at the 5′-end and a fluorescent dye quencher, TAMRA (6-carboxytetramethyl-rhodamine), at the 3′-end. Quantitative RT-PCR was performed with the TaqMan universal PCR master mix (Applied Biosystems) at 50°C for 2 min and 95°C for 10 min, then run for 40 cycles at 95°C for 15 s and 60°C for 1 min, and analyzed on an MJ research detection system (MJ Research 2002; Opticon monitor version 2.10.10). Results were expressed as the NIS copy numbers normalized against a standard curve of levels of the internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA obtained from complementary DNA fragments that were coamplified in each PCR run. We performed 2 independent experiments in duplicate.
For Western blot analyses, cells were harvested and protein extracts were prepared in lysis buffer (50 mmol/L N-(2-hydroxyethyl)piperazine-N′- (2-ethanesulfonic acid), 0.15 mol/L NaCl, 0.5% Nonidet P-40) containing the protease inhibitors (phenylmethylsulfonylfluoride, tosyl-lysylchloromethyl-ketone, and tosylphenylalanyl-chloromethane). The lysates were separated by 10% reduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to a polyvinyldifluoride membrane filter, which was then blocked with 5% nonfat dry milk in Tris-buffered saline Tween (TBST) buffer (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.1% Tween 20) for 30 min at room temperature. Western blot analysis was then performed by incubating the filter with a mouse monoclonal antihuman NIS antibody (mAb-3564; Chemicon) (26) in TBST containing 5% nonfat dry milk at 4°C overnight, followed by incubation with peroxidase-conjugated goat antirabbit IgG (1:3,000) for 60 min at room temperature. Immunodetection was performed using the Pierce enhanced-chemiluminescence system (Amersham) for 1 min exposed to x-ray film.
For immunocytochemical staining, cells were fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 30 min at room temperature. After washing with phosphate-buffered saline, 2% bovine serum albumin was added as a blocking reagent and cells were incubated for 60 min at room temperature. The cells were then serially incubated with a primary mouse monoclonal antihuman NIS antibody (mAb-3564; Chemicon) and a secondary Cy-3−labeled antimouse antibody (The Jackson Laboratories). The number of NIS-expressing cells was estimated visually under a fluorescence microscope and quantified using a fluorescence-activated cell sorter (FACScan; Becton-Dickinson). We performed 2 independent experiments using MCF-7 cells with and without adenovirus-mediated NIS transfer.
Radioiodine Uptake Assay
Radioiodine uptake was determined at steady-state conditions as described by Weiss et al. (27). In brief, MCF-7 cells were plated in a 24-well plate at a cell density of 1 × 106 cells per well and cultured. For the radioiodine uptake assay, 10 μmol/L of NaI and 3.7 kBq of 125I (New England Nuclear) in 0.5 mL of Hanks' buffered salt solution were added to each well, and cells were incubated for 1 h at 37°C. The specific activity under these conditions was 740 MBq/mmol. After incubation, the media were removed, the cells were washed twice with 1 mL of ice-cold Hanks' buffered salt solution, and 95% ethanol was added at room temperature. After an additional 20 min, 0.5 mL of the supernatant from each well was recovered and counted in a γ-counter (Cobra II; Packard). For the sodium perchlorate inhibition study, 30 μmol/L of sodium perchlorate were added to each well 20 min before 125I administration, and the same uptake assay was performed. Data represent the absolute amount of accumulated 125I inside cells (pmol/106 cells). On the basis of the growth inhibition studies with retinoic acid, each experiment to determine cell numbers was performed separately in triplicate as described.
Data Analysis
Numeric data were expressed as mean ± SD. Data comparisons between groups were performed by the nonparametric Kruskall–Wallis test or the repeated-measures ANOVA test. A P value < 0.05 was considered statistically significant.
RESULTS
Efficiency of Gene Transfer
The MCF-7 cells were efficiently transduced with Rad-LacZ. At 5 MOI infection of Rad-LacZ, β-galactosidase expression on X-Gal staining was about 10%. However, at 50 MOI infection, most of the cells expressed β-galactosidase, and the transduction efficiency was estimated to be >60% (Fig. 1).
Efficiency of adenovirus-mediated gene transfer in MCF-7 cells shown by X-Gal staining. Blue-colored cells represent expression of β-galactosidase. Efficiency of gene transfer is >60% at 50 MOI. MI = mock infection.
Cell Growth Inhibition by ATRA
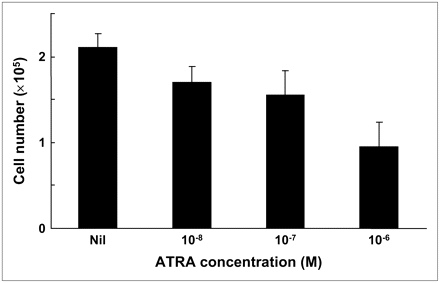
Proliferation of MCF-7 cells was inhibited in a dose-dependent manner by exposure to ATRA at concentrations of 10−8–10−6 mol/L (P < 0.05; Fig. 2). After a 48-h incubation without ATRA, the mean cell number per well was 2.10 × 105, whereas after 48-h incubation in the presence of 10−6 mol/L of ATRA, the mean cell number was 0.95 × 105.
Effect of ATRA on growth inhibition of MCF-7 cells. Cellular proliferation was inhibited by ATRA in a dose-dependent manner over 10−8–10−6 mol/L concentration range (P < 0.05).
Enhancement of NIS Gene Expression by ATRA
Quantitative RT-PCR showed an increase in mRNA expression with ATRA treatment (P < 0.001) (Fig. 3). Untreated MCF-7 cells showed a small amount of basal NIS mRNA expression (4,550 ± 1,202 copies/μg of GAPDH). With 10−6 mol/L ATRA treatment, the mRNA level was increased by 2.1-fold compared with wild-type MCF-7 cells (9,450 ± 212 copies/μg of GAPDH). By contrast, the increase in mRNA expression with ATRA was more marked in Rad-NIS–infected cells. The expression levels in these cells were 21.6-fold (98,500 ± 707 copies/μg of GAPDH) greater than those in wild-type MCF-7 cells. After incubation with 10−6 mol/L ATRA, the mRNA level in Rad-NIS–infected cells was increased to 118.5-fold that of wild-type MCF-7 cells (539,000 ± 45,255 copies/μg of GAPDH; P < 0.001).
Results of quantitative real-time RT-PCR. NIS mRNA expression was increased 2.1-fold with ATRA treatment in wild-type MCF-7 cells, whereas NIS expression increased by 118.5-fold in adenovirus-infected MCF-7 cells. Enhancement effect of ATRA is more marked in adenovirus-infected cells.
Western blot analysis and immunocytochemical staining showed similar results. Levels of the main immunoreactive protein in MCF-7 cells, which has a molecular mass of ∼90 kDa, increased with ATRA treatment combined with adenovirus-mediated NIS transfer (Fig. 4). In Rad-NIS– infected cells, there was a significant, dose-dependent increase in NIS protein expression levels with ATRA treatment. However, wild-type MCF-7 cells showed only a low level of NIS expression even with ATRA treatment. The immunocytochemical staining and flow cytometry analysis also showed similar results (Figs. 5 and 6). MCF-7 cells that had not been infected with Rad-NIS showed only faint immunoreactivity (not shown), whereas cells infected with Rad-NIS showed marked plasma membrane staining with some intracellular immunoreactivity. Strong immunoreactivity was observed in MCF-7 cells with 10−6 mol/L ATRA treatment (Fig. 5). In wild-type MCF-7 cells, the numbers of NIS-positive cells were 0.2% ± 0.1%, 0.2% ± 0.1%, 0.3% ± 0.2%, and 0.5% ± 0.4% with 0, 10−8, 10−7, and 10−6 mol/L ATRA treatment, respectively, whereas those in Rad-NIS–infected MCF-7 cells were 17.6% ± 1.9%, 20.2% ± 2.1%, 38.8% ± 2.5%, and 56.6% ± 3.3%, respectively (Fig. 6).
Effect of ATRA treatment on NIS protein expression in MCF-7 cells assessed by Western blot analysis. Main immunoreactive protein in MCF-7 cells, ∼90 kDa, increased with ATRA treatment combined with adenoviral gene transfer. ATRA significantly increased expression in dose-dependent manner in cells infected with adenoviruses carrying NIS. However, wild-type MCF-7 cells showed only faint NIS expression despite ATRA treatment. hNIS = human NIS.
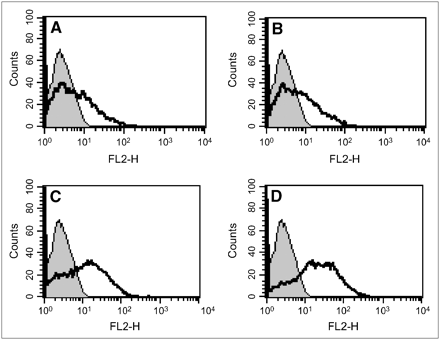
MCF-7 cells with adenovirus-mediated NIS gene transfer showed prominent plasma membrane staining with intracellular immunoreactivity after ATRA treatment (A–D), whereas wild-type MCF-7 cells revealed only faint staining (not shown). (A) No ATRA. (B) 10−8 mol/L ATRA. (C) 10−7 mol/L ATRA. (D) 10−6 mol/L ATRA.
MCF-7 cells with adenovirus-mediated NIS transfer showed increasing numbers of fluorescent positive cells after ATRA treatment (A–D), whereas wild-type MCF-7 cells showed only minimal increases in fluorescently labeled cells with ATRA treatment (not shown). (A) No ATRA. (B) 10−8 mol/L ATRA. (C) 10−7 mol/L ATRA. (D) 10−6 mol/L ATRA. FL2-H = 585/42 filter-height of fluorescence intensity.
Radioiodine Uptake Assay
ATRA treatment increased 125I uptake in a dose-dependent manner (P < 0.001) (Fig. 7). Untreated MCF-7 cells showed only small levels of 125I uptake (8.9 ± 1.1 pmol/106 cells). The uptake increased by 3.1-, 5.5-, and 7.6-fold with 10−8, 10−7, and 10−6 mol/L ATRA, respectively. Rad-NIS–infected cells showed a 4.0-fold increase in 125I uptake (35.4 ± 1.1 pmol/106 cells), and treatment with 10−8, 10−7, and 10−6 mol/L ATRA increased 125I uptake by 4.9-, 8.2-, and 27.6-fold compared with wild-type MCF-7 cells. Rad-NIS–infected MCF-7 cells treated with 10−6 mol/L ATRA showed the greatest levels of 125I uptake in this study (245.0 ± 13.7 pmol/106 cells), which was markedly greater than the sum of the levels observed with ATRA treatment and Rad-NIS infection alone. Increased 125I uptake was completely blocked by treatment with 30 μmol/L KClO4 (Fig. 7).
Effect of ATRA on radioiodine uptake in MCF-7 cells. 125I uptake in wild-type MCF-7 cells increased by 3.1-, 5.5-, and 7.6-fold with treatment of 10−8, 10−7, and 10−6 mol/L ATRA, respectively. Treatment with 0, 10−8, 10−7, and 10−6 mol/L ATRA in Rad-NIS–infected cells increased 125I uptake by 4.0-, 4.9-, 8.2-, and 27.6-fold greater than that in wild-type MCF-7 cells. Rad-NIS–infected MCF-7 cells with 10−6 mol/L ATRA treatment (245.0 ± 13.7 pmol/106 cells) is much greater than the sum of levels seen with ATRA treatment and Rad-NIS infection alone. RA = retinoic acid.
DISCUSSION
The present study demonstrates that ATRA treatment increases adenovirus-mediated transgene expression in an estrogen receptor–positive MCF-7 cell line. NIS mRNA, protein levels, and radioiodine uptake were all greater in Rad-NIS–infected MCF-7 cells treated with ATRA than in MCF-7 cells treated with only ATRA. Combined treatment with ATRA and adenoviral gene delivery resulted in levels of NIS gene expression and radioiodine uptake that were greater than the sum of the levels observed with ATRA treatment and adenoviral NIS delivery alone.
The action of retinoic acid is mediated through specific nuclear receptors known as retinoic acid receptors (RARs) and retinoid X receptors (RXRs). These 2 receptor subfamilies interact with cis-acting DNA-binding sites (RAREs) in the promoter regions of target genes as either heterodimeric RXR/RAR or homodimeric RXR/RXR and act as ligand-dependent transcription factors (28). The endogenous NIS gene has RAREs in its promoter region (29), so these receptors are thought to affect the ability of NIS to respond to retinoic acid. In cases of breast cancer, increased NIS expression and growth inhibition with retinoic acid were observed only in the estrogen receptor–positive cancers (8,10,30). The RARs in MCF-7 cells include abundant RARα, RARγ, and RXRα, as well as some RARβ and RXRβ (6,31). It has been reported that increased expression of the NIS gene with ATRA treatment is likely to be mediated by RAR (8,10). The ATRA–RAR complex activates RAR/RXR heterodimers and then binds to retinoic acid– responsive genes (32). The present study confirms the results of earlier reports that retinoic acid can increase radioiodine uptake in MCF-7 cells (8–13).
RAR/RXR activation has also been shown to modulate adenovirus-mediated transgene expression (20). Characterization of the human CMV promoter showed that selective signaling of RARs by retinoic acid was dominant in the retinoic acid–response pathway for activating human CMV (19). In a transfection experiment with an adenovirus-mediated CMV-driven gene, only cells transfected with RAR/RXR expression vectors demonstrated marked enhancement in adenovirus-mediated gene expression with retinoic acid treatment (19). In our study, we transferred the human CMV-driven NIS gene to MCF-7 breast cancer cells. As RARs, RXRs, and estrogen receptors are expressed in MCF-7 cells (21), the increased expression of the CMV-driven NIS transgene was expected due to direct activation of the RAREs in CMV by retinoic acid–specific receptors. The results of this study demonstrate the enhancement effects of retinoic acid on adenovirus-mediated NIS gene and protein expression, as well as on radioiodine uptake in MCF-7 cells.
In this study, the increases in NIS gene and protein expression, and in radioiodine uptake in MCF-7 cells after ATRA treatment, were less than those in Rad-NIS–infected MCF-7 cells treated with ATRA. The discrepancy in mRNA expression may be due to the time period over which levels of ATRA-induced and adenovirus-mediated mRNA expression were measured. We measured NIS mRNA and protein expression in MCF cells 72 h after ATRA treatment, when the ATRA-induced radioiodine uptake and adenovirus-mediated NIS might have reached maximum levels. Retinoic acid–induced mRNA expression has been reported to reach a maximum after 12 h but then decreases to <40% of the maximum level after 72 h (8,10). Adenovirus-mediated NIS mRNA expression was determined 48 h after adenoviral infection on the basis of previous studies with an adenoviral vector (33,34).
Western blot analysis and immunocytochemical staining showed only faint NIS expression in ATRA-treated wild-type MCF-7 cells. On the other hand, a 7.6-fold increase in radioiodine uptake was observed with 10−6 mol/L ATRA treatment. The amounts of radioiodine uptake were similar to those observed in Rad-NIS–infected MCF-7 cells in this study and were mostly consistent with the results from previous studies (8,9,13). The low level of induction of NIS protein expression with retinoic acid has also been observed in previous studies in MCF-7 cells (8,9,12). Although Kogai et al. detected a major deglycosylated NIS band by Western-blotting analysis in ATRA-treated MCF-7 cells (8,9), a large amount of protein (150 μg) was applied (35), and the NIS protein band was not normalized for β-actin. In addition, ATRA induced only some immunoreactivity at the plasma membrane (9). These results, together with those from our study, indicate that ATRA induces only a low level of functional NIS protein expression at the plasma membrane. The magnitude of radioiodine uptake found in the present study in ATRA-treated MCF-7 cells is greater than that reported by Unterholzner et al. (12). The discrepancy is thought to be due to the use of a different strain of MCF-7 cells (12).
The results obtained in this study conclusively demonstrate the increase in adenovirus-mediated transgene expression induced by retinoic acid. Our findings correspond with an earlier study (20), although the level of transgene expression induced by retinoic acid alone in this previous study seems to be lower than that observed in our study. This is probably because the previous study assessed the effect of retinoic acid in cells without retinoic receptor expression. In this study, the cytotoxic effects of radioiodine in MCF-7 cells were not assessed. Confirmation of these in vitro effects in animals with tumors is needed to support the use of systemic retinoic acid therapy in breast cancer models.
A further increase in adenovirus-mediated NIS gene expression in MCF-7 cells may be achieved by a combination of retinoic acid and other transcriptionally active drugs. Gaetano et al. demonstrated that a combination of retinoic acid and trichostatin A, a histone-deacetylase inhibitor, increased and prolonged adenovirus-mediated transgene expression in infected tissues and cells (20). In addition, combined RXR stimulation may also increase adenovirus-mediated transgene expression (10). Joint activation of RAR and RXR contributes to transactivation (19), although signaling of RAR by retinoic acid plays an important role in modulating CMV infection (36). The heterodimerization of RXR with RAR stabilizes binding of the receptors to their responsive elements and promotes ligand-dependent transcriptional regulation (37). Therefore, the combination of ATRA and an RXR-specific ligand may be more effective. Dexamethasone is another candidate for combination therapy. In MCF-7 breast cancer cells, treatment with a combination of ATRA and dexamethasone resulted in synergistic enhancement of endogenous NIS expression and radioiodine-induced cytotoxicity (11,12). The stabilization of RXR-RAR by dexamethasone may contribute to ligand-dependent transcription of adenovirus-mediated transgene, although there is no direct evidence for adenovirus-mediated gene expression. Finally, increased effectiveness of retinoic acid stimulation may also be achieved by more selective retinoid stimulation (11). Further studies assessing the effect of combining other nuclear receptor ligands may provide additional insights into the role of retinoic acid on adenovirus-mediated transgene upregulation.
CONCLUSION
This study demonstrated increased expression of adenovirus-mediated NIS expression in MCF-7 cells after retinoic acid treatment. Increased NIS expression with ATRA treatment was identified at the levels of mRNA, protein, and radioiodine uptake. Our results indicate that improved efficiency of NIS gene therapy or reporter imaging in breast cancer may be possible with retinoic acid treatment. The results of this study can be applied to other therapeutic or reporter gene transfer trials. The expression of adenovirus-mediated transgenes can be enhanced by retinoic acid and appropriate cellular combinations of retinoic acid nuclear receptors. Combined treatment with other transcriptionally active drugs may further increase the induction of adenovirus-mediated gene therapy or imaging. It may also allow transgene induction with a lower dose of retinoic acid in vivo, while reducing retinoic acid toxicity.
Acknowledgments
This study was supported by grant 02-032 from the Asan Institute for Life Sciences, Seoul, Korea.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication August 20, 2006.
- Accepted for publication November 28, 2006.