Abstract
PET is now widely used in the diagnosis and staging of lung cancer with 18F-FDG. The purpose of the study was to evaluate the prognostic value of diffuse bone marrow hypermetabolism along with other PET prognostic factors with respect to survival and compare them with other established prognostic factors in a large cohort of patients. Methods: Of 255 patients referred for evaluation of a suspicious lung lesion by PET over an 8-mo period (May 1999 to January 2000), the outcome of 120 patients with a final diagnosis of primary non–small cell lung cancer was analyzed retrospectively after excluding subjects with benign, metastatic, or recurrent lesions, using the available follow-up information and a provincial mortality database. Kaplan–Meier survival curves were compared using the mean and the maximal tumor standardized uptake value (SUV), bone marrow SUV, PET stage, various laboratory parameters, sex, age, conventional imaging stage, and pathologic stage. A stepwise Cox proportional hazard model was built using the significant variables on univariate analysis. Results: The primary tumor SUV (>10), bone marrow uptake of 18F-FDG, 18F-FDG PET stage, pathologic stage, hypercalcemia, lactate dehydrogenase, hemoglobin, albumin, thrombocytopenia, thrombocytosis, and leukocytosis were predictors of mortality on univariate analysis. On multivariate analysis, bone marrow hypermetabolism, 18F-FDG PET nodal stage, and some hematologic parameters (hemoglobin, platelets, white blood cell counts) remained significant independent predictors of mortality. Conclusion: Bone marrow hypermetabolism and the PET nodal stage were strong independent predictors of mortality in patients with lung cancer. The primary tumor SUV, though predictive on univariate analysis, was not an independent predictor of mortality in our model.
In industrialized countries, lung cancer has become the most common malignant tumor. Despite recent advances in treatment regimens, the 5-y prognosis of non–small cell lung carcinoma (NSCLC) remains dismal. Even for patients with localized disease, survival is relatively poor, with 5-y survival rates as low as 61% and 38% for stage 1a and stage 1b disease, respectively (1). Reliable and readily available prognostic factors could hopefully provide a method to identify subgroups of patients most likely to benefit from complementary therapeutic options such as neoadjuvant or adjuvant therapy (either chemotherapy or radiation therapy) (2). Similarly, for patients with advanced disease, the use of adequate prognostic factors could help differentiate patients who could benefit from more aggressive chemotherapeutic regimens from those in whom no significant response is to be expected (3).
According to an extensive review of the literature published recently by Brundage et al. (4), >150 prognostic factors have been reported for NSCLC. Among them, TNM classification has been consistently shown to be the most powerful. Several other factors—clinical (weight loss, performance status, sex, age), biochemical (hypercalcemia, albumin, lactate dehydrogenase [LDH]), hematologic (hemoglobin), or molecular markers—have been used as well, with variable power and consistency.
18F-FDG PET as a whole, in relation to its accurate staging abilities (5–9), has already been shown to be an excellent prognostic factor in NSCLC patients (10). In addition, many authors have already demonstrated that the metabolic activity of the primary NSCLC lesion, as measured by the tumor standardized uptake value (SUVT), can also be used as a prognostic factor (11–17). However, the cutoff values that have been used vary considerably, ranging from 5 to 20. The SUV of the primary lesion has been related to the pathologic aggressiveness of pulmonary adenocarcinomas as well (18). The relationship between SUV and histologic type of NSCLC is controversial as one author described significant differences in SUVs between NSCLC subtypes while another found no significant difference.
In our clinical experience, we noticed that some patients, who were referred for 18F-FDG PET in the setting of pulmonary nodule evaluation or staging of NSCLC, showed unexplained, diffusely increased bone marrow metabolic activity (Fig. 1). We also observed that this subgroup of patients seemed to have a very poor outcome, which appeared to be even worse than that of the average patient with NSCLC (19). The objective of this study was to evaluate the prognostic significance of PET parameters relating to the tumor SUV, stage of disease on PET, and bone marrow activity and to assess their independence relative to some well-known prognostic factors. We also searched for potential causal factors that could explain bone marrow hypermetabolism.
Coronal, sagittal, and transaxial slices (left to right) through bone marrow of a patient with NSCLC showing markedly increased bone marrow metabolism on 18F-FDG PET. Qualitatively, bone marrow activity is remarkably greater than mean liver activity.
MATERIALS AND METHODS
Patients
Records of the first 255 patients who were referred for evaluation of a lung lesion from May 1999 to January 2000 of a lung nodule, staging of a proven pulmonary malignancy or assessment of recurrence were reviewed retrospectively. Patients who were referred for evaluation of a possible or proven recurrence (n = 37) were excluded from the survival analysis. We also excluded patients whose final diagnosis revealed small cell lung carcinoma or metastatic lesions (n = 16) from another primary, patients with lesions considered benign on 18F-FDG PET (n = 45), and patients with normal PET scans (n = 29). Of the 14 patients with equivocal results on 18F-FDG PET, only those with a proven final diagnosis of NSCLC were included (n = 6). A total of 120 patients were included in the analysis. Mortality data were obtained from hospital records as well as from the Quebec provincial mortality database (Institut de la Statistique du Québec).
Data Acquisition
Whole-body 18F-FDG PET scans were acquired on a dedicated PET scanner (Ecat HR+; Siemens) from the neck to the pelvis. Patients were required to be fasting for a minimum of 6 h. Blood glycemia was monitored with a portable capillary glucometer, and a small bolus of intravenous insulin was administered as needed in a few patients with blood glucose levels above 8 mmol/L. A minimal delay of 1 h occurred between the intravenous injection of insulin and 18F-FDG administration. All subjects received a 18F-FDG dose of 7 MBq/kg. The mean delay from 18F-FDG injection to imaging was 91 min. Images were acquired in 2-dimensional mode (with septa) for 8–10 min per bed position and were reconstructed with an iterative algorithm (ordered-subset expectation maximization; 2 iterations, 16 subsets) with and without attenuation correction. The attenuation map was obtained with a 68Ge transmission source.
The mean and maximum SUVT was readily available as they are routinely reported at the Centre Hospitalier Universitaire de Sherbrooke. The maximum SUVT is obtained by drawing a region of interest (ROI) over the most intense slice of the primary lesion. The mean SUVT was obtained by drawing a ROI whose borders are defined by an automatic isocontour set at 75% of the maximum SUV within the ROI. (The 75% cutoff used for the mean SUV allowed for good reproducibility between subjects and excluded regions of tumor necrosis in order to obtain a measurement most representative of the metabolically active part of the lesions.)
A mean SUV was also obtained for each of the 3 larger homogeneous vertebrae visualized in the field of view (most of the time, L3, L4, and L5, unless showing severe osteoarthritic changes or metastasis). A ROI was drawn over the vertebral body, again using an automatic isocontour ROI set at 75% of the maximum SUV. The bone marrow SUV (BM SUV) was defined as the mean value of the 3 selected vertebrae. Normal reference values for BM SUV (mean ± SD, 1.32 ± 0.23) and for the relative ratio of activity between bone marrow and liver (BM/L) (mean ± SD, 0.94 ± 0.26) were obtained from a group of 20 healthy subjects, without any evidence or past history of oncologic or hematologic disease. All of these reference patients were free of disease after a minimum of 5-y follow-up. A ROI was also drawn on a homogeneous transaxial slice of the liver to obtain the BM/L ratio. All SUVs obtained were corrected for weight and lean body mass (45.5 + 0.91 × [height (cm) − 152]).
Statistical Analysis
Statistical analysis was performed on S-Plus 6.0 (Insightful Corp.). Univariate Kaplan–Meier survival analyses were performed on SUVT, BM SUV, BM/L, and 18F-FDG stage as well as on the accepted prognostic factors that were available from patient records (age, sex, hemoglobin, LDH, calcium, albumin, conventional imaging stage, pathologic stage, and other hematologic parameters such as platelet and white blood cell [WBC] count). In addition to chest radiography and chest CT, conventional imaging included, as needed, abdominal ultrasound, brain CT or MRI, and bone scintigraphy. Some well-known prognostic factors, such as weight loss and performance status, could not be assessed retrospectively from this database as they were not collected systematically.
All variables were dichotomized using cutoffs reported in the literature when available or the best discriminating value as determined by recursive partitioning. Variables shown to be of prognostic significance (defined as a log-rank test P value < 0.05) on univariate survival analysis (Kaplan–Meier) were then entered into a Cox proportional hazards model using 2 different approaches: stepwise forward selection and backward deletion (20). In forward stepwise selection, the variable with the strongest association with mortality on univariate analysis is entered first, followed by the next strongest until all variables that are significant (at a prespecified level; in this case, P < 0.05 on log-rank test) are entered into the model. Variables entered in the model that become no longer significant are deleted sequentially. In backward deletion, all variables significant on univariate analysis are entered into the model and are sequentially deleted starting with the variable having the weakest association with mortality in the model until all of the variables left are significant at a prespecified level (P < 0.05). Variables related to the pathologic stage were not entered into multivariable models considering that this information is usually not available in clinical practice at the time of decision making and that a considerable portion of our population was composed of patients with advanced stage disease, who were not treated surgically and for whom this information was therefore missing.
Finally, we looked for some possible associations between bone marrow hypermetabolism (increased BM SUV and BM/L ratio) and variables that could presumably be causally related to it. For continuous variables, the Spearman correlation test was used (as BM SUV did not have a normal distribution). For the evaluation of discrete variables, patients were subdivided into 2 subgroups—that is, normal BM versus bone marrow hypermetabolism, according to either their BM SUV or BM/L ratio, using the best discriminating cutoff value found in survival analysis. Variables were then tested for statistical independence using the Fisher exact test.
RESULTS
Our sample population was composed of 120 patients (41 females, 79 males), aged 34–89 y (median age, 68 y). They were all referred for evaluation of lung lesions, for which the final diagnosis was NSCLC. The follow-up period ranged from 12 d to 81 mo (median, 18.5 mo). The median follow-up for patients officially still alive from the data available was 63 mo. Eighty-three deaths occurred in this follow-up interval. More details on population characteristics are available in Table 1.
Patient Characteristics
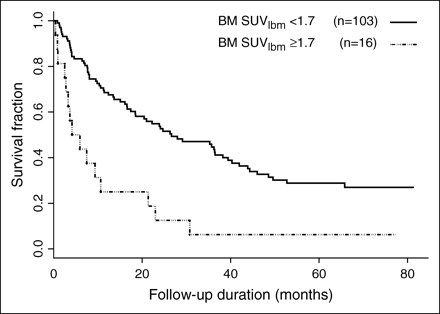
The variables that were significant in Kaplan–Meier analyses are summarized in Table 2, along with their P values. The results confirmed our previous clinical impression with regard to the possible association of bone marrow hypermetabolism with a dismal prognosis (Fig. 2). Patients with a BM/L ratio of >1.5 had a significantly shorter survival than patients with a lower BM/L ratio (6 vs. 24 mo; P = 0.00006, log-rank test). The absolute value of BM SUV corrected for lean body mass was as powerful a predictor of mortality (P = 0.00004, log-rank rank test) as BM/L.
Kaplan–Meier survival curves of NSCLC patients with bone marrow hypermetabolism (BM SUV > 1.7) on 18F-FDG PET vs. patients with normal bone marrow metabolism. BM SUV is normalized for lean body mass (lbm). Median survival of patients with bone marrow hypermetabolism was significantly shorter than that of NSCLC patients with normal bone marrow metabolism (151 vs. 799 d; P = 0.00006, log-rank test). BM = bone marrow.
Kaplan–Meier Analysis: Univariate Survival Analysis
As expected, the mean SUVT also proved to be a significant prognostic factor in our population (Fig. 3), using a cutoff value of 10 as reported by Ahuja et al. (11). The median survival of patients with a mean SUVT of >10 was 8 mo compared with 29 mo for those with an SUV of <10 (P = 0.003, log-rank test).
Kaplan–Meier survival curves of NSCLC patients with high tumor SUV (weight-corrected mean SUVT > 10) on 18F-FDG PET vs. patients with less active primary tumors. Median survival of patients with elevated SUVT was significantly shorter (227 vs. 874 d; P = 0.003, log-rank test). T = primary tumor.
Among SUVT, BM/L ratio, and BM SUV in the subgroup of patients who were treated surgically, only the BM/L ratio remained significant, whereas all of these factors were highly significant in nonsurgically treated patients (Table 3).
Kaplan–Meier Analysis According to Treatment
The most powerful predictors among all types of dichotomized staging variables were the 18F-FDG nodal stage (stage N0–N1 vs. stage N2–N3) (P = 0.002, log-rank test) and the pathologic stage (stage 1–2 vs. stage 3–4) (P = 0.001, log-rank test). On the other hand, the performance of conventional modalities for predicting mortality was much less powerful, as none of the different dichotomized T, N, and M factors or stages reached significance because of the design of the study.
Among the clinical and biochemical factors, sex (P = 0.003, log-rank test) and age (<60 y old; P = 0.02, log-rank test) were relatively good mortality predictors in our study. Hypocalcemia (P = 0.02), LDH (P = 0.002), hemoglobin (P = 0.000001), and albumin (P = 0.02, log-rank test) were all shown to be significant predictors of mortality, although, for hemoglobin, a cutoff value of 90 g/L obtained by recursive partitioning was used instead of the usual 110 g/L cutoff.
Survival analysis of some other parameters of the whole-blood count presumably related to bone marrow hypermetabolism revealed unexpectedly that thrombocytopenia (<160 × 1012/L) was the most powerful predictor of mortality of all factors studied (P = 0.0000002). To our knowledge, only 1 study reported thrombocytopenia as a significant predictor of mortality, in patients with advanced small cell lung cancer (21). Thrombocytosis (P = 0.01) and leukocytosis (P = 0.03) showed a weaker association with mortality. Detailed results of all prognostic factors that proved significant and that were included in the multivariate analysis are summarized in Table 2.
Multivariate survival analysis was realized using the 2 different approaches described earlier. In our set of patients, both methods happened to converge to the same model (Table 4), which included 6 variables: bone marrow hypermetabolism (expressed as BM SUV corrected for lean body mass), nodal stage on 18F-FDG PET (stage N0–N1 vs. stage N2–N3), anemia (hemoglobin, <90 g/L), thrombocytopenia (<150 × 1012/L), thrombocytosis (>340 × 109/L), and leukocytosis (WBCs) (>12.5 × 109/L). BM SUV turned out to be more strongly associated with mortality than the BM/L ratio on multivariate analysis. Surprisingly, according to this model, bone marrow hypermetabolism would be independent of many whole-blood count abnormalities that proved to be significant on univariate analysis. Unlike what was reported previously, the SUV of the primary lesion did not prove to be an independent prognostic factor in our study.
Cox Model: Multivariate Analysis
Analysis of parameters potentially associated with bone marrow hypermetabolism revealed that both BM SUV and BM/L correlated weakly to moderately with the WBC level and less strongly with the platelet level (positive correlations). BM SUV also correlated moderately with the arterial Pao2 level (partial pressure of oxygen, arterial; expressed as percentage of the expected level) and correlated weakly with the hemoglobin level (negative correlations) and SUVT (positive correlation). Association of bone marrow hypermetabolism with the T stage, N stage, or global stage was rather inconsistent. Spearman correlation coefficients and Fisher exact test P values are summarized in Table 5.
Correlation Between SUVT, BM Hypermetabolism, and Potential Causal Factors
DISCUSSION
As we suspected from our clinical experience, bone marrow hypermetabolism proved to be an excellent predictor of mortality. In fact, it was among the most significant prognostic factors that could be evaluated in this study, second only to thrombocytopenia and anemia on univariate analysis. It was more significant than the SUV of the primary lesion and even more powerful than any parameter related to the stage of disease. It is worth noting that the BM SUV had be corrected for lean body mass instead of actual weight because BM SUV corrected for weight was not a significant prognostic factor of survival due to spuriously elevated BM SUVs in obese patients.
The metabolic activity of the primary lesion was again confirmed to be a predictor of mortality in this study. However, it was not independent of other predictors on multivariate analysis. In previous studies in which it was reported to be an independent prognostic factor, the only factors that remained significant in the Cox models along with SUVT were the stage of disease in 3 studies (11,14,15) and the stage of disease plus performance status in another study (12).
18F-FDG PET staging was also confirmed to be an accurate predictor of mortality. The predictive value of the nodal stage determined on 18F-FDG PET was comparable with the overall pathologic stage and appeared slightly better than the nodal stage determined surgically, but many patients in this series did not undergo extensive surgical sampling. In this study, all prognostic factors not directly related to 18F-FDG PET suffered from a selection bias. With the patient selection being based on a 18F-FDG PET scan database, the PET-related data were more likely to be complete than non-PET–related variables. Some patients had their CT scans in remote clinics or hospitals and their results were incomplete or missing. This has certainly reduced the relative prognostic value of conventional staging. Also, because the clinicians based their investigation largely on PET scan results, conventional imaging staging was much less extensive than it would have been without 18F-FDG PET. Thus, the lower prognostic significance of conventional imaging compared with 18F-FDG PET could be somewhat artificial. In this patient population, not all lesions reported as suggestive of metastasis on conventional imaging were investigated, because other clinical parameters or test results, including PET, might have led investigators to conclude that the likelihood of metastasis was low. Because a conventional stage had to be assigned without the benefit of the PET results, such findings were considered to indicate metastatic disease on conventional staging, even when further investigation was unavailable. The heterogeneity of the subjects relative to the stage of disease might also have reduced the significance of some prognostic factors that are more specific to either localized disease or advanced disease.
Another limitation of this multivariate analysis is the absence of 2 of the most important clinical parameters in NSCLC—weight loss and performance status, which were not systematically recorded in patients' records. Therefore, the results of this analysis must be interpreted with caution. In fact, they are probably appropriate for evaluating the relative value of 18F-FDG PET factors between themselves but they are certainly less accurate for evaluating their relative value compared with clinical factors. A prospective study in which all data are systematically collected could solve many of these problems.
The etiology of bone marrow hypermetabolism observed in some NSCLC patients is unclear. Among the 16 patients with elevated BM SUV, none had received granulocyte colony-stimulating factor (G-CSF). Only 1 patient had a previous history of hematologic disease (myeloproliferative syndrome) and another had a previous history of endometrial cancer. The remaining 12 patients had no previous history of cancer or hematologic disease. A bone marrow aspiration performed on 1 patient with diffuse, but not focal, marrow hypermetabolism showed reduced erythroblasts with otherwise normal cellularity and iron reserves, without evidence of metastatic or proliferative disease. Four of the patients with diffuse marrow hypermetabolism also had focal bone metastases, and another patient developed multiple bone metastases within 6 mo after diagnosis.
Among the possible explanations for bone marrow hypermetabolism are secretion of stimulating cytokines by the primary tumor and invasion of bone marrow by micrometastases. Cytokines such as colony-stimulating factors and interleukin-6 (IL-6) (22) as well as vascular endothelial growth factor (VEGF) (23) can be secreted by tumor cells. Both leukocytosis and thrombocytosis have been associated with NSCLC (24–26) and even with poor prognosis (27–32). Interestingly, a case of diffuse bone uptake of 201Tl attributed to G-CSF secretion by a large cell carcinoma was reported previously (33). The abnormal uptake disappeared after tumor resection.
With special immunocytochemistry techniques, bone marrow micrometastases and lymph node micrometastases can be detected in up to 31%–60% of NSCLC patients without nodal involvement (34–36), but this has not always been shown to be of prognostic significance (37). Such immunocytochemistry techniques were not applied to our patients with increased BM SUV.
Further studies are thus required to better identify the exact cause of increased BM SUV. Such studies should include serum levels of cytokines such as VEGF, G-CSF, granulocyte–macrophage colony-stimulating factor, and IL-6 as well as immunocytochemistry cytokeratin staining of some bone marrow aspirates. Confirmation of any or both of these hypotheses would certainly provide an additional argument to support administration of neoadjuvant or adjuvant chemotherapy.
Bone marrow hypermetabolism is possibly also related to factors depending on the host rather than on the tumor itself: Other variables that were significantly associated with BM SUV include the Pao2 and the hemoglobin level. The hemoglobin and Pao2 levels represent relative hypoxemic states that can more-or-less directly stimulate hemopoiesis. Bone marrow hypermetabolism could also be influenced by coexisting illnesses indirectly or completely unrelated to cancer.
CONCLUSION
Bone marrow hypermetabolism appears to be a strong predictor of mortality in NSCLC on univariate analysis. Further studies are required to clarify its etiology. An elevated primary tumor SUV (>10) was shown once again to be a predictor of mortality in NSCLC. The N factor based on 18F-FDG PET was the most important stage-related prognostic factor in our study.
Our Cox model revealed that bone marrow hypermetabolism and the presence of nodal metastases on 18F-FDG PET were prognostic factors independent of anemia, thrombocytopenia, thrombocytosis, and leukocytosis; these results remain to be confirmed in a prospective study that would include the systematic collection of well-recognized factors such as performance status and weight loss. The primary tumor SUV was not an independent prognostic factor from the parameters used in this model.
References
- Received for publication September 12, 2005.
- Accepted for publication December 28, 2005.