TO THE EDITOR:
We read with great interest the recent article by Wessels et al. (1). The article purports to propose a clinically relevant, standard method for the blood-based estimation of red marrow absorbed dose for radiolabeled antibody therapy as a benchmark for intercomparison purposes. We believe that the proposed model contains a mathematic error and that, even if corrected, the method may have limited clinical relevance.
The Mathematic Error: Treatment of Remainder-of-Body Component
The absorbed dose to red marrow due to 131I activity in the remainder tissues of the body is given as (1):
 where:
where:




This S value differs from that given in the article because an incorrect value for S(RM ← TB)MD11 was used, leading to a miscalculation. More important, contrary to their stated intent, the authors’ approach has resulted in a remainder-of-body S value that is patient mass independent, when it should be mass dependent.
In order to add the necessary mass dependence, the 2 S values themselves must be mass adjusted (2). Each S value should be multiplied by MRM–MD11/MRM–patient, which may be approximated by MWB–MD11/MWB–patient, leading to a multiplicative factor of 70/MWB–patient. Therefore:
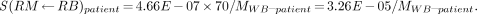
It has previously been shown that ignoring this mass dependence may lead to significant calculational errors (2).
Establishing a Clinically Relevant, Standard Method for Red Marrow Dosimetry
Although most institutions have adopted the 2-component approach to red marrow dose calculation, the methods used are only similar, not identical. Wessels et al. (1) suggest that this lack of standardization caused many investigators to use a dosing metric based on administered activity, when in reality these nondosimetric methods have been proven effective. Although variations in dosimetry methods will certainly contribute uncertainty to red marrow dose estimates, the small sample set (consisting of datasets containing only 2 patients for 6 of the 7 participating institutions) included in the article (1) makes it difficult to comprehend the true range of uncertainty, and therefore the reported “benchmarking” is of limited value.
There are 3 major mathematic features that require standardization in the blood-based estimation of red marrow absorbed dose. We have already discussed the remainder-of-body S value; the other 2 are conversion of blood data to red marrow data, and phantom choice.
Conversion of Blood Data to Red Marrow Data. A commonly used, practical method to estimate the ratio of cumulated activity concentration in red marrow to that in blood uses the term red marrow extracellular fluid fraction/(1 − hematocrit), as proposed in the article (1). The red marrow extracellular fluid fraction is assumed to have a constant value of 0.19, a value obtained from a study of the albumin space in the red marrow of rabbit femur and, importantly, a value not intended for use in patients whose marrow has been compromised by therapy (3). Because the majority of patients receiving radiolabeled monoclonal antibody therapy (using either commercially available or investigational agents) have undergone prior therapies resulting in vastly differing marrow reserves and radiosensitivities, assigning a constant value, such as 0.19, may not be a clinically relevant approach because it does not adequately address these other variables in a manner to reduce the variation in hematologic toxicity that is generally encountered.
Phantom Choice. MIRD Pamphlet No. 11 S values and phantom masses (4) for 131I were used in the article (1). Patients treated with 186Re were included in the investigation, but the phantom choice was not specified for these patients (186Re does not appear in MIRD Pamphlet No. 11). A review of the literature indicates that more investigators have used MIRDOSE (5) than MIRD Pamphlet No. 11. It would seem more reasonable to use MIRDOSE, or potentially OLINDA (organ level internal dose assessment) (6), S values and phantom mass values to accommodate a standard method for both 131I and 186Re.
In conclusion, we believe that the clinically relevant standard method for the blood-based estimation of red marrow absorbed dose presented by Wessels et al. (1) contains a mathematic error and uses a phantom that does not include both radionuclides presented in the article. Thus, the method can hardly be viewed as a benchmark standard. We do, however, strongly endorse the idea that marrow dosimetry should be standardized, but in terms of not only parameters of absorbed dose but also parameters of marrow status (i.e., the ability of individual patients’ marrow to tolerate additional myelosuppressive treatment), especially in heavily pretreated patient populations (7,8).
REFERENCES
REPLY:
With the exception of the specific points considered below, the primary concerns raised by Siegel et al. center on use of the word benchmark. As the title of the paper indicates, its focus was a multiinstitutional comparison (1). As such, the conclusions of the paper remain valid. Regarding the specific points:
The correspondents have noted that a value used in Table 1 differs from their own calculation. The discrepancy lies in the number of significant figures used in the calculation. In converting to SI units and using S(RM ← WB) to derive S(RM ← RB), my coauthors and I rounded the published MIRD Pamphlet No. 11 (2) value of 1.1E−05 to 1E−05. The difference in the values for S(RM ← RB) is, therefore, due to a truncation error resulting in an overall 3%–7% underestimate in dose values calculated for institutions using 131I in Table 2. When other errors involved in dosimetry calculations are considered, the conclusions drawn from Table 2 remain unchanged. The correct Table 1 values for S(RM ← WB) and S(RM ← RB) are 8.261E−07 and 4.660E−07 mGy/MBq-s, respectively.
As has been previously recognized in MIRD Pamphlet No. 11 (2), there is no simple approach to mass scaling of an S factor that includes combined electron and photon contributions, such as S(RM ← WB). Although phantom models and S values have been updated in MIRDOSE3.1 and organ level internal dose assessment (OLINDA) codes (3,4), no systematic study has been published that provides us with further information on how to perform this scaling. In fact, MIRD Pamphlet No. 11 indicates that for target organs sufficiently distant from source organs, one would expect the specific absorbed fraction and the S value to be independent of mass (i.e., increases in patient size result in compensating increases in both organ mass and cross-organ photon absorbed fraction). MIRD Pamphlet No. 11 states that the photon contribution from the target self-dose varies as M–2/3 and electron self-dose as M–1. The scaling suggested by Siegel et al. and used by Stabin et al. (5) has yet to be validated to support the approach of applying a linear mass correction (M−1) to the photon cross-dose contribution. Notably, strict adherence to the guidance provided by MIRD Pamphlet No. 11 regarding mass correction was properly applied in the OLINDA code (4). OLINDA documentation cites an earlier Snyder publication (6) in which the particulate component of the self-dose varies as M−1, photon self-dose varies as M−2/3, and photon cross dose is mass independent. Hence, the mass correction methodology used in the present work remains consistent with MIRD Pamphlet No. 11 recommendations. It remains the method of choice pending new studies using variable-mass patient phantoms that would substantiate any further change in patient mass scaling. Nevertheless, if such a mass correction were to be included in the cross-dose term (Eq. 11) of the article and at a power of M–1, the result would be a 2%–6% change to any of the values appearing in Table 1. This change is similar to the change one would arrive at for using lean body mass and less than if MIRDOSE3 S values were used. However, the text used to describe Equations 9–11 in the article is unclear and a clarification will be included in an erratum. Large uncertainties noted by Siegel et al. are dominated by the electron contribution from the self-dose term for the physically relevant data presented in the article and were appropriately mass corrected in Equation 8. This is discussed by Shen et al. (7), for whom a mathematically correct derivation showed an apparent canceling of mass dependence for the self-dose term when the blood concentration was used. One is reminded that the dominance of a mass adjustment for the self-dose term remains because blood concentration automatically scales this term to be patient specific.
Regarding sample size, we certainly would have preferred to analyze a greater number of patients per institution. However, total patient number (n = 21) is well within nominal standards that have been traditionally used for MIRD dose estimate reports. As noted in the article, a greater number of patients were submitted by all institutions. Several of these patients, however, did not meet our inclusion criteria for data entry into the study or remain to be analyzed. Because the emphasis of the paper is on an interinstitutional comparison of calculations relative to a standardized approach given the same dataset, the most critical n value is the number of institutions. The reproducibility of calculation methodology at any individual institution is a secondary to the specific aims as stated above. We had 7 institutions participating and believe that the conclusions of the paper are not invalidated by the sample number for each institution.
Comments regarding variations in red marrow extracellular fluid fraction, MIRDOSE S values, prior therapies, and marrow reserve have already been extensively addressed in this article. As Siegel et al. point out and as was discussed in our paper, it is important to understand the limitations of the blood-based remainder-of-body dosimetry formulation. The correspondents are correct. 186Re S value was adopted from MIRDOSE3.1. A similar approach was taken by the contributing institution but using MIRDOSE2.
To summarize, Siegel et al. have identified errors in the article that lead to at most 10% differences in the final published results, well within the error associated with radionuclide dosimetry calculations. Nevertheless, corrections will be published via an erratum. Suggested mass corrections to the cross-dose term do not agree with MIRD Pamphlet No. 11 methodology and the OLINDA program and have no experimental basis supported in the literature. It is our opinion that this paper makes a meaningful contribution and that the validity of the conclusions is inconsistent with the concerns raised in the letter.
REPLY:
I respectfully dissent from the majority opinion offered regarding point 2 in the response of Wessels to the letter of Siegel et al. I remain in agreement with the position that “with measured blood activity concentration, red marrow dose may be represented by a patient mass-independent term, involving red marrow self dose, and a patient mass-dependent term, involving dose from the remainder of the body” (1). This position is also shared by Shen et al. (2) and was reiterated recently by Siegel (3). I believe this approach to be the most correct and accepted current method to perform patient-specific corrections to standardized marrow dose calculations. The article of Wessels et al. (4) treats both terms as being patient mass independent and thus treats all patients as if they are of standard size and mass. Correct accounting for the electron and photon contributions from activity in the remainder of the body is important, especially when the cumulated activity ratios for remainder of body to red marrow are high. This may result in differences in calculated marrow dose from standardized models of much larger than 2%–6%, as claimed by Wessels in his response; our calculations indicated that patient-specific marrow doses might be different from those calculated using standard phantoms by 20%–70% (Table 2 of Stabin et al. (1)). The arguments raised by Wessels in his response to eliminate the patient specificity of the second term, regarding mass-based corrections to absorbed fractions, apply most correctly to discrete (not distributed) organs in the body. Whether this type of correction can be applied to values of S(RM ← RB) could be tested and might produce some interesting results for future study. I do not, however, believe that a sufficient scientific basis exists to support this argument (and I tried to point this out to the other authors in the spring of 2003). I enthusiastically applaud the efforts of the authors to encourage multiinstitutional comparisons of marrow dose in order to study dosimetry and possible dose–effect relationships. I suggest, however, that if patient-specific modifications are undertaken, they be applied with consistent and widely accepted methods and include patient-specific corrections for target region masses when possible.







