Abstract
3-123I-Iodo-α-methyltyrosine (123I-3-IMT) is used for the detection of residual and recurrent brain tumors. The application of 123I-3-IMT for the study of extracerebral malignancies is limited by its marked and rapid renal uptake. In this study, we compared the tumor uptake, biodistribution, and specificity of 5 structurally related iodinated amino acids with those of 123I-3-IMT. The aim was to select the optimal analog for oncologic imaging outside the brain. Methods: We studied 3-123I-iodotyrosine (123I-3-IT), 2-123I-iodotyrosine (123I-2-IT), 123I-iodo-azatyrosine (123I-IAzaT), 2-123I-iodophenylalanine (123I-2-IPhe), and 4-123I-iodophenylalanine (123I-4-IPhe). Tumor uptake and renal uptake in sarcoma-bearing rats were measured by use of in vivo dynamic imaging. The differential uptake ratio (average counts per pixel of the region of interest divided by the average counts per pixel inside the total body) and rates of tracer accumulation (K1 values) were calculated. Results were compared with the values obtained for 123I-3-IMT in the same rat. Tracers that demonstrated high tumor uptake were labeled with 125I and coinjected with 18F-FDG in rats with turpentine-induced acute inflammation. After 30 min, the rats were sacrificed and dissected. Amino acid tracer uptake in organs and tissues was measured, and the increase in uptake in the inflamed muscle was expressed relative to the increase in 18F-FDG uptake. Results: Tumor uptake and K1 values for 123I-2-IT and 123I-2-IPhe were comparable to those for 123I-3-IMT. 123I-4-IPhe showed high tumor uptake but a reduced K1 value because of high blood-pool activity. 123I-3-IT and 123I-IAzaT did not accumulate markedly in tumor tissue. Renal accumulation of 123I-2-IT, 123I-2-IPhe, and 123I-4-IPhe was at least 6 times lower than that of 123I-3-IMT. 18F-FDG uptake was markedly increased in areas of acute inflammation (215%). The increases for 125I-3-IMT and 125I-4-IPhe were 35.5% and 22.2%, respectively, of the increase for 18F-FDG. Almost no increase was found for 125I-2-IT (3.3%) and 125I-2-IPhe (2.8%). Conclusion: 123I-2-IT and 123I-2-IPhe are promising tracers for oncologic imaging outside the brain. 123I-2-IT has the advantage of an established kit for radiosynthesis.
Amino acid tracers are mainly used for the study of brain tumors. High tumor uptake and low physiologic uptake in gray matter result in clear images that surpass those obtained with 18F-FDG (1,2). The accumulation reflects the increased amino acid transport (AAT) activity of cancer cells (3). A few reports have demonstrated that certain amino acid tracers are accumulated less by inflammatory cells, suggesting that increased AAT accumulation is more tumor specific than increased glucose accumulation (4–6). Such specificity is of utmost importance for the study of residual and recurrent tumor tissues after primary therapy. Therefore, it would be interesting to study AAT status by use of PET or SPECT of suggestive lesions in the follow-up after therapy (7). However, amino acid imaging of extracerebral tumors has not been explored extensively (8,9).
3-123I-Iodo-α-methyltyrosine (123I-3-IMT) is the main tracer used to study AAT by SPECT. Its application in the abdominal area is limited by its marked and rapid renal accumulation. The aim of this study was to compare 5 structurally related iodinated amino acids and to select the analog with the best characteristics for the study of residual or recurrent malignant disease outside the brain. The parameters used were tumor uptake, biodistribution, and uptake into inflammatory tissue. Tumor uptake and biodistribution for the analogs were compared with those for 123I-3-IMT. Uptake of the analogs in areas of inflammation was compared with that of 18F-FDG.
MATERIALS AND METHODS
Laboratory Animals
For the tumor model, male Wag/Rij rats (n = 5) were subcutaneously injected in the right flank with 106 R1M rhabdomyosarcoma cells (Harlan Nederland). Tumors were grown for 4 wk. For the inflammation model, male Wag/Rij rats (n = 12) were injected in the left calf muscle with 0.15 mL of turpentine. Two rats with a tumor were also injected with turpentine for a combined tumor–inflammation model. Inflammation experiments were performed 24 h after turpentine injection. The animals had free access to water and food until 4 h before tracer injection. The animals were anesthetized with halothane. Afterward, the animals were sacrificed by intravenous injection of KCl. The study protocol was approved by the ethical committee for animal studies of our institution, and National Institutes of Health principles of laboratory animal care (NIH publication 86-23, revised 1985) were followed.
Radiopharmaceuticals
123I- or 125I-3-IMT.
Radioiodination of l-α-methyltyrosine (Sigma-Aldrich) with 123I or 125I (Nordion Europe) was performed by carrier-added electrophilic substitution with IODO-GEN (Pierce Europe) as an oxidizing agent. The reaction was performed at 0°C, and the reaction mixture was mixed for 2 min, yielding >90% 123I- or 125I-3-IMT. 123I- or 125I-3-IMT was separated form the starting molecule by use of a small reverse-phase C18 Sep-Pak column (Waters) as described by Gühlke and Biersack (10). A radiochemical purity of >99% was obtained.
3-123I-Iodotyrosine (123I-3-IT) and 123I-Iodo-Azatyrosine (123I-IAzaT).
Radioiodination and purification of l-3-iodotyrosine and l-iodo-azatyrosine (Sigma-Aldrich) were performed as described for 123I-3-IMT, yielding >95% 123I-3-IT and >98% 123I-IAzaT. A radiochemical purity of >99% was obtained.
2-123I- or 125I-Iodotyrosine (123I- or 125I-2-IT).
Radioiodination of 0.5 mg of l-2-iodotyrosine (ABX) was performed by Cu+-assisted nucleophilic exchange under acidic and reducing conditions (0.5 mg of SnSO4, 5 mg of gentisic acid, 12 mg of citric acid, and 0.325 mg of CuSO4·H2O) at 120°C for 60 min, yielding >98% 123I- or 125I-2-IT and a radiochemical purity of >98%.
4-123I- or 125I-Iodophenylalanine (123I- or 125I-4-IPhe).
Radioiodination of l-4-iodophenylalanine (Sigma-Aldrich) was performed by Cu+-assisted nucleophilic exchange as described above, yielding 98% 123I- or 125I-4-IPhe and a radiochemical purity of >98%.
2-123I- or 125I-Iodophenylalanine (123I- or 125I-2-IPhe).
Radioiodination of 0.5 mg of l-2-bromine-phenylalanine was performed by Cu+-assisted nucleophilic exchange as described above, yielding >98% 123I- or 125I-2IPhe. Purification by semipreparative reverse-phase high-pressure liquid chromatography (C18 column [250 × 10 mm]; 5% acetonitrile–95% H2O–0.1% trifluoroacetic acid; 6 mL/min) followed by preconcentration in and recovery from a C18 Sep-Pak column cartridge resulted in a final radiochemical purity of >98%.
The chemical structures of the iodinated amino acids are shown in Figure 1.
Chemical structures of iodinated amino acid analogs under investigation.
18F-FDG.
18F-FDG was produced by nucleophilic fluorination.
Dynamic Planar Imaging
Tumor, renal, and blood-pool activities as functions of time for the various 123I-labeled amino acid analogs were measured by dynamic imaging with a gamma camera equipped with a medium-energy collimator (the resolution was 11.1 mm at full width at half maximum). Imaging was started immediately after intravenous injection of 18.5 MBq of the appropriate tracer. A total of 240 images of 10 s each were acquired in 128 × 128 matrices with a zoom factor of 3.2 (pixel size, 1.5 mm) and a photopeak window set at 15% around 159 keV. In order to reduce variability between different animals, we investigated each rat with 123I-3-IMT (reference) and 1 or 2 of the other tracers on different days. Images were corrected for remaining 123I activity from the previous day by use of a static acquisition of 5 min before tracer injection.
Regions of interest (ROIs) were manually drawn around the tumor, the contralateral background area, the left ventricle of the heart, the right kidney, and the total body. The injected dose was defined as the total number of counts inside the total body. Tracer uptake was calculated as the differential uptake ratio (DUR; average counts per pixel in the ROI divided by average counts per pixel inside the total body) and plotted as a function of time. For comparison of the various tracers, the area under the curve (AUC) was calculated for the DUR curves between 10 and 40 min after injection. The ratio of the AUC for the tested amino acid tracer to the AUC for 123I-3-IMT obtained in the same rat was calculated for comparison. Similarly, ratios of tumor uptake to background uptake were plotted as a function of time and, for comparison, AUC ratios were calculated.
Patlak Analysis
A Patlak analysis of the data from the first 7 min, carried out by use of the counts inside the heart as the input function, was performed for the calculation of rates of tracer uptake (K1 values) for tumor accumulation and renal accumulation by use of the following equation:
 The graph of this equation yields a straight line with a slope equal to the K1 value (11,12). For renal accumulation, T(t) is substituted by R(t). The ratio of the K1 value for the tested amino acid tracer to that for 123I-3-IMT obtained in the same rat was calculated for comparison.
The graph of this equation yields a straight line with a slope equal to the K1 value (11,12). For renal accumulation, T(t) is substituted by R(t). The ratio of the K1 value for the tested amino acid tracer to that for 123I-3-IMT obtained in the same rat was calculated for comparison.
Biodistribution and Accumulation into Areas of Inflammation
Tracers that demonstrated high tumor accumulation in the imaging study were selected for the biodistribution study carried out by use of Wag/Rij rats with turpentine-induced inflammation. The animals were injected with 5 MBq of one of the 125I-labeled amino acid tracers together with 37 MBq of 18F-FDG and then sacrificed at 30 min after injection. The organs and tissues were removed rapidly, washed, and weighed. Muscle tissues were collected from the inflamed left leg and noninflamed right leg. The radioactivity of the samples was counted by use of a γ-counting system (Cobra Inspector 5003; Canberra Packard). 18F activity was counted on day 1, and 125I activity in the same samples was counted 3 d later. The amount of radioactivity in the tissue was expressed as the differential absorption ratio (activity per gram of sample divided by the activity injected per gram).
The increase in accumulation in the inflamed muscle versus the noninflamed muscle was calculated. The increase in 18F-FDG accumulation for individual rats was regarded as a measure of the degree of induced inflammation. The increase in the accumulation of the amino acid tracers was expressed as a percentage of the increase in the uptake of 18F-FDG in the same rat.
Dynamic PET Imaging
Two rats with coexisting tumor and turpentine-induced inflammation were studied. The first rat underwent a dynamic planar imaging study after 123I-3-IMT injection and then a dynamic 18F-FDG PET study. The second rat underwent a dynamic planar imaging study after 123I-2-IT injection and then a dynamic 18F-FDG PET study. Dynamic planar imaging was performed as described above. PET imaging was performed immediately after intravenous injection of 10 MBq of 18F-FDG with an LSO-PET camera (ACCEL-Siemens; the resolution was 6.4 mm at full width at half maximum). A total of 40 images of 60 s each (40 min) were acquired. Images were reconstructed iteratively (32 subsets, 10 iterations), and all coronal slices were reprojected in 2 dimensions for image quantification. ROIs were drawn over the inflamed muscle, the contralateral noninflamed muscle, the tumor, and the total body. The DUR as a function of time was calculated for the different ROIs. The DUR in inflamed muscle versus that in noninflamed muscle was plotted as a function of time for the amino acid and 18F-FDG studies.
Statistical Analysis
We studied the behavior of different tracers in the same rat, always including a reference tracer (123I-3-IMT or 18F-FDG) to reduce variability between different animals (tumor size and amount of inflammation). The results are expressed as the average and the SD or range as appropriate.
RESULTS
Dynamic Planar Imaging
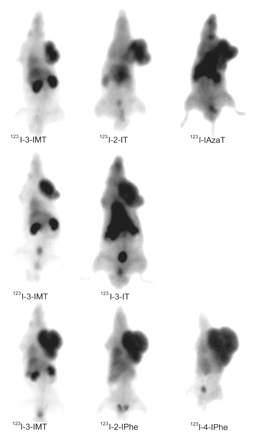
Tumor uptake of 123I-2-IT, 123I-2-IPhe, and 123I-4-IPhe was comparable to that of 123I-3-IMT (Table 1). 123I-3-IT and 123I-IAzaT tumor uptake was low. Renal activity was high for 123I-3-IMT but considerably lower for the other amino acid tracers. Blood-pool activity was highest for 123I-4-IPhe and 123I-IAzaT. The rate of tracer entering the tumor (K1 value) for 123I-2-IT and 123I-2-IPhe was comparable to that for 123I-3-IMT but was 37% lower for 123I-4-IPhe. The correlation coefficients (R2) of the Patlak analysis ranged from 0.91 to 0.98. The ratio of tumor uptake to background uptake for 123I-2-IT was closest to that for 123I-3-IMT. From this experiment, we selected 123I-2-IT, 123I-2-IPhe, and 123I-4-IPhe for further study; 123I-3-IT and 123I-IAzaT were rejected because of their low tumor accumulation. The summed image (between 30 and 40 min after injection) for the 6 different tracers is shown in Figure 2.
Summed images (30–40 min after injection) from dynamic planar imaging study. Images are scaled to maximum in tumor.
Comparison of Tested Amino Acid Tracers with 123I-3-IMT (Reference)
Biodistribution and Accumulation into Areas of Inflammation
Comparative biodistribution data for 125I-3-IMT, 125I-2-IT, 125I-2-IPhe, 125I-4-IPhe, and 18F-FDG are shown in Table 2. Marked pancreatic accumulation was measured for all amino acid tracers. Brain activity was high for 18F-FDG but low for the amino acid tracers. Renal activity was very marked for 125I-3-IMT but was at least 6 times lower for 125I-2-IT, 125I-2-IPhe, and 125I-4-IPhe. Liver activity was comparable for the 4 amino acid tracers. Blood-pool activity was highest for 125I-4-IPhe.
Biodistribution of 4 Amino Acid Analogs and 18F-FDG in Wag/Rij Rats
The increase in tracer accumulation in inflamed muscle versus noninflamed muscle was highest for 18F-FDG (average, 215%). Relative to the increase in 18F-FDG accumulation, the increases in amino acid accumulation in inflamed muscle were 35.5% (range, 15.6%–57.7%) for 125I-3-IMT, 3.3% (range, 0.3%–6.2%) for 125I-2-IT, 2.8% (range, 2.9%–7.6%) for 125I-2-IPhe, and 22.2% (range, 16.9%–26.8%) for 125I-4-IPhe.
Dynamic PET Imaging
The images of the 123I-2-IT and 18F-FDG PET studies of an R1M tumor-bearing rat with coexisting inflammation are shown in Figure 3. The ratios of tracer uptake in inflamed tissue to that in noninflamed tissue as a function of time for 18F-FDG/123I-3-IMT and 18F-FDG/123I-2-IT are shown in Figure 4. The ratio was initially higher for 123I-3-IMT than for 18F-FDG and stayed nearly constant as a function of time. The ratio was significantly lower for 123I-2-IT than for 18F-FDG and decreased as a function of time. The tumor accumulation of 18F-FDG was higher than that for the amino acid tracers; the DUR values were 1.43 for 18F-FDG/123I-3-IMT and 1.08 for 18F-FDG/123I-2-IT.
18F-FDG and 123I-2-IT images from R1M tumor-bearing rat with coexisting inflammation (summed images at 30–40 min after injection).
Ratio of tracer uptake in inflamed tissue to that in noninflamed tissue as function of time for 18F-FDG (+) and 123I-3-IMT (•) (A) and for 18F-FDG (+) and 123I-2-IT (▪) (B).
DISCUSSION
We compared the biodistribution data for 6 radioiodinated amino acid analogs by using in vivo dynamic imaging. In order to avoid variability between animals, different tracers were studied in the same rat and always with a reference tracer (123I-3-IMT or 18F-FDG). Tracers with high tumor accumulation were further investigated by dissection studies. The data showed that apparently small molecular modifications of the amino acid tracers resulted in important differences in biodistribution. The position of the iodine atom appears to be critical; a tyrosine molecule with an iodine atom placed in the ortho position (123I-2-IT) is markedly accumulated by a tumor, whereas tumor uptake is low when iodine is situated in the meta position (123I-3-IT). However, when a methyl group is added to the α-carbon atom of 123I-3-IT to yield 123I-3-IMT, the molecule is once again accumulated in a tumor. The undesirable marked renal accumulation of 123I-3-IMT is also linked to the α-carbon methyl group, as 123I-3-IT is only weakly captured by the kidneys.
The uptake of 123I-2-IT, 123I-2-IPhe, and 123I-4-IPhe was high in tumor tissue and low in the kidneys. However, the blood-pool activity of these tracers was higher than that of 123I-3-IMT, especially for 123I-4-IPhe. High blood-pool activity for 123I-4-IPhe has been reported before (13,14) and reduced the ratio of tumor uptake to background uptake in our study. Again, the structural analog with the iodine in the ortho position (123I-2-IPhe) has a more favorable biodistribution. The data suggest that the relatively large iodine atom induces the least amount of steric hindrance in the ortho position, preserving high-affinity recognition by AAT system L (see below). Because the affinity (Km) of the amino acid tracers for the specific transporters is in the micromolar range and because endogenous levels of competing substrates are also in the micromolar range, we can exclude the possibility that nanomolar differences in tracer carrier concentrations attributable to different labeling methods had a significant influence on the quantitative measurements in our study.
As with the differences in biodistribution, we also found important differences in specificity. The acute inflammation induced by turpentine consists of a large number of granulocytes (15).125I-2-IT and 125I-2-IPhe did not accumulate markedly in areas of acute inflammation, whereas 125I-3-IMT and 125I-4-IPhe showed significant accumulation. For 125I-4-IPhe, this accumulation could have been caused by its high blood-pool activity. The reason for the significant 125I-3-IMT accumulation in areas of inflammation is less clear. Nonspecific uptake of 125I-3-IMT has been reported before (8,16,17). A possible explanation could involve the transporter subtype selectivity of the different analogs; AAT system L represents not a single transporter protein but a small family of transporter proteins (LAT1, LAT2, and others) with small differences in substrate recognition (18). The differential expression of system L transporter subtypes on tumor cells and inflammatory cells was recently described (19). We speculate that different structural analogs prefer different transporter subtypes and that this characteristic may explain the important differences in biodistribution and specificity between 123I-3-IMT and 123I-2-IT or 123I-2-IPhe. Therefore, it may not be correct to state that all radiolabeled amino acids are tumor specific; this characteristic depends on the type of transporter protein that is studied with the selected tracer.
Tumor accumulation of 18F-FDG measured by PET was higher than tumor accumulation of 123I-3-IMT and 123I-2-IT measured by use of a gamma camera. This finding can be attributed partially to the lower resolution of the gamma camera, leading to an underestimation of tracer accumulation.
Some deiodination, demonstrated by thyroid uptake, was found on the images of 123I-IAzaT. Metabolite or protein incorporation was not studied here. Previous experiments showed that 123I-3-IMT and 123I-2-IT are not metabolized and do not enter protein synthesis (20). The absence of metabolism of these iodinated analogs also has been attributed to the iodine atom, because analogs labeled with the much smaller fluorine atom do enter intracellular metabolic pathways and are incorporated into proteins (21).
CONCLUSION
123I-2-IT and 123I-2-IPhe are both promising tracers for oncologic imaging outside the brain because of their high tumor accumulation, low renal uptake, and minor accumulation into areas of acute inflammation. Because of low physiologic brain uptake, these tracers are also interesting for the detection of brain tumors. The 123I-2-IT analog could be the best tracer for clinical studies because its chemical synthesis is relatively easy and can be performed with a kit formulation.
Acknowledgments
This work was supported by a grant from the Belgian Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO-Vl).
Footnotes
Received Jan. 16, 2003; revision accepted Apr. 21, 2003.
For correspondence or reprints contact: Tony Lahoutte, MD, Department of Nuclear Medicine, Academic Hospital, Free University Brussels (AZ-VUB), Laarbeeklaan 101, 1090 Jette, Belgium.
E-mail: tony.lahoutte{at}az.vub.ac.be