Abstract
To our knowledge, no study investigating the usefulness of cardiac PET for detection of myocardial involvement of sarcoidosis is available. We investigated whether 13N-NH3/18F-FDG PET could identify cardiac involvement in patients with sarcoidosis. Methods: Seventeen patients with cardiac sarcoidosis underwent cardiac 13N-NH3/18F-FDG PET under fasting condition. Systemic sarcoidosis was diagnosed by histologically proven noncaseating epithelioid granuloma, and cardiac sarcoidosis was diagnosed according to the Japanese Ministry of Health and Welfare guidelines for diagnosing cardiac sarcoidosis. Results: Only 6 patients exhibited myocardial 201Tl defects and only 3 patients exhibited abnormal 67Ga accumulation in the heart. Thirteen patients exhibited 13N-NH3 defects, and 14 patients exhibited increased 18F-FDG uptake in the heart; 12 patients exhibited both 13N-NH3 defects and increased 18F-FDG uptake, 2 patients exhibited increased 18F-FDG uptake but no 13N-NH3 defect, and 1 patient exhibited 13N-NH3 defects but no increased 18F-FDG uptake. 13N-NH3 defects were observed frequently in the basal anteroseptal wall of the left ventricle, and increased 18F-FDG uptake was observed frequently in the basal and midanteroseptal-lateral wall of the left ventricle. Involvement of the apex was rare. Seven patients were treated with steroid hormone and underwent follow-up cardiac PET 1 mo after steroid therapy. 13N-NH3 defects exhibited no significant change after steroid therapy, whereas increased 18F-FDG uptake was markedly diminished in size and intensity in 5 patients and disappeared completely in 2 patients. Conclusion: Our findings suggest that cardiac 13N-NH3/18F-FDG PET is the most useful method both for the identification of cardiac involvement of sarcoidosis and for the assessment of cardiac sarcoidosis disease activity.
Sarcoidosis is a multisystem disorder of unknown etiology. Although the organ most frequently affected is the lung, all parts of the body can be affected. Overall prognosis is good because organ involvement is usually asymptomatic and the disease is often self-limiting (1). Cardiac sarcoidosis, however, sometimes causes fatal ventricular tachyarrhythmias, conduction block, and left ventricular (LV) dysfunction (2,3) and may lead to a poor prognosis. Myocardial involvement is present in at least 25% of patients with systemic sarcoidosis (3), and sudden death due to ventricular tachyarrhythmias or conduction block is responsible for 30%–85% of deaths from sarcoidosis (2). Endomyocardial biopsy may be essential for establishing the diagnosis of cardiac sarcoidosis. However, it is invasive and may be insensitive because myocardial involvement is not homogeneous (2,4). 201Tl, 67Ga (1,2,5–7), and 123I-metaiodobenzylguanidine scintigraphy (8) and magnetic resonance imaging (9) are also used to detect cardiac involvement in patients with sarcoidosis. Areas with 201Tl defects are considered areas of fibrogranulomatous replacement, although 201Tl defects in the myocardium are not specific to sarcoidosis and may occur with ischemic heart disease or other cardiomyopathies (1,2,5). Accumulation of 67Ga is considered an indicator of inflammatory change, and 67Ga scintigraphy is useful in diagnosis of cardiac sarcoidosis and in prediction of effects of steroid therapy (5).
Recent studies revealed that 18F-FDG in PET accumulated in the lung and bilateral hilar lymph nodes in patients with sarcoidosis (10,11). Moreover, in patients with pulmonary sarcoidosis, 18F-FDG uptake of the lung was concordant with histologic activity in lung and was decreased after high-dose steroid therapy (10). It is thus possible that areas of fibrogranulomatous replacement in the heart may show increased 18F-FDG uptake and that 18F-FDG PET may provide a means of assessment of disease activity of pulmonary sarcoidosis. However, to our knowledge, no study investigating the usefulness of cardiac PET for detection of myocardial involvement of sarcoidosis is available, with the exception of one case report (12).
In this study, we investigated whether 13N-NH3/18F-FDG PET could identify cardiac involvement in patients with sarcoidosis.
MATERIALS AND METHODS
Patients
We retrospectively identified 17 patients (4 men, 13 women; mean age, 58 ± 12 (±SD) y; range, 29–72 y) in our patients’ database with cardiac sarcoidosis who underwent cardiac 13N-NH3/18F-FDG PET. Systemic sarcoidosis was diagnosed by histologically proven noncaseating epithelioid granuloma with giant cells, and cardiac sarcoidosis was diagnosed according to the Japanese Ministry of Health and Welfare guidelines for diagnosing cardiac sarcoidosis (13) described in Table 1. Characteristics of the 17 patients with cardiac sarcoidosis are summarized in Table 2.
Guidelines for Diagnosis of Cardiac Sarcoidosis from Japanese Ministry of Health and Welfare
Characteristics of 17 Patients with Cardiac Sarcoidosis
PET
For PET imaging, a Shimadzu-SET 1400 W-10 PET scanner (HEADTOME IV; Shimadzu Corp.) was used. This scanner can obtain 7 slices simultaneously with a 13-mm interval, a slice thickness of 11-mm full width at half maximum (FWHM), and a spatial resolution of 4.5-mm FWHM. Axial, 6.5-mm-interval Z-motion of the scanner provides 14 contiguous transverse slices of the myocardium.
A 10-min transmission scan was obtained using a rotating 68Ge rod source. The acquired data were used to correct emission images of 13N-NH3 and 18F-FDG for body attenuation. After completion of the transmission scan, the patient remained in the supine position and was injected intravenously with 555–740 MBq 13N-NH3. After a 5-min delay to allow pulmonary background activity to clear, myocardial perfusion imaging was performed for 10 min.
After at least 5 h of fasting and 3–4 h after completion of the perfusion scan, the patient received an intravenous injection of 259–370 MBq 18F-FDG. Fifty minutes were allowed for cardiac uptake of 18F-FDG. Imaging of glucose utilization was then performed for 10 min.
Images were collected in 256 × 256 matrices and reconstructed using Butterworth and ramp filters along the short axis, horizontal axis, and vertical long axis of the heart by a computer system (Dr. View; Asahi-Kasei Joho System Co.).
The LV myocardium was divided into 9 segments (basal anterior, midanterior, basal septal, midseptal, basal inferior, midinferior, basal lateral, midlateral, and apex). Each short-axis slice of the LV was divided into 36 sectors, 10° each, and a bull’s eye polar map was reconstructed from the short-axis slices extending from the base to the apex. The maximal count in the LV was selected, and the value of each pixel was normalized to a maximal count of 100 in 13N-NH3 and 18F-FDG images. The mean values of 13N-NH3 (% 13N-NH3) and 18F-FDG (% 18F-FDG) counts in each segment were calculated.
Analysis of PET Images
The short-axis, horizontal-axis, and vertical long-axis images were normalized to the maximum count in each image set and were displayed as color scale images. Two experienced nuclear cardiologists visually interpreted 13N-NH3 and 18F-FDG uptake in 9 LV segments and in the right ventricular free wall. 13N-NH3 defects were defined as definitely decreased 13N-NH3 uptake (% 13N-NH3, approximately <60%). A myocardial segment with normal 13N-NH3 uptake and minimal % 18F-FDG was considered as a normal control segment. In the segments with a 13N-NH3 defect, 18F-FDG uptake equal to or higher than that in the normal control segment was defined as increased. In the segments without a 13N-NH3 defect, definitely higher 18F-FDG uptake than that in the normal control segment was defined as increased. Segmental 18F-FDG uptake index quantitatively indicating the degree of increase in 18F-FDG uptake was calculated as follows: 18F-FDG uptake index = % 18F-FDG of each segment/% 18F-FDG of the normal control segment.
201Tl and 67Ga Scintigraphy
Eleven patients underwent myocardial 201Tl SPECT, and 15 patients underwent whole-body 67Ga planar imaging, 9 of whom underwent myocardial 67Ga SPECT. Experienced nuclear cardiologists visually interpreted 201Tl defects, and experienced nuclear radiologists visually interpreted 67Ga accumulation unaware of the findings of cardiac PET.
Statistics
Values are given as mean ± SD. The % 13N-NH3 or the 18F-FDG uptake index before and after steroid therapy were compared with the paired t test.
RESULTS
Findings of myocardial 201Tl and 67Ga scintigraphy and cardiac PET in 17 patients with cardiac sarcoidosis are summarized in Table 3. Six patients exhibited myocardial 201Tl defects and 3 patients exhibited abnormal 67Ga accumulation in the heart. The 67Ga accumulation in the heart in the latter 3 patients was observed in both planar and SPECT images. Thirteen patients (76%) exhibited 13N-NH3 defects, and 14 patients (82%) exhibited increased 18F-FDG uptake in the heart; 12 patients exhibited both 13N-NH3 defects and increased 18F-FDG uptake, 2 exhibited increased 18F-FDG uptake but no 13N-NH3 defect, and 1 exhibited 13N-NH3 defects but no increased 18F-FDG uptake. Only 2 patients (12%) (patients 1 and 2) exhibited neither 13N-NH3 defect nor increased 18F-FDG uptake. Five patients showed significant uptake of 18F-FDG in hilar or mediastinal lymph nodes. 13N-NH3 defects were observed frequently in the basal anteroseptal wall of the LV, and increased 18F-FDG uptake was observed frequently in the basal and midanteroseptal-lateral wall of the LV. Both 13N-NH3 defects and increased 18F-FDG uptake were rare in the apex. Of 14 patients with increased 18F-FDG uptake in the heart, 13 underwent 67Ga scanning, of whom only 3 (23%) exhibited abnormal 67Ga accumulation in the heart.
Results of Visual Analysis in Myocardial 201Tl and 67Ga Scintigraphy and Cardiac PET
Seven patients were treated with steroid hormone and underwent follow-up cardiac PET 1 mo after steroid therapy. On visual analysis, 13N-NH3 defects exhibited no significant change after steroid therapy, whereas increased 18F-FDG uptake was markedly diminished in size and intensity in 5 patients and disappeared completely in 2 patients (patients 10 and 15). The % 13N-NH3 in 13 LV segments with 13N-NH3 defects before steroid therapy did not differ significantly after steroid therapy (42.0% ± 6.7% before steroid therapy vs. 45.0% ± 7.0% after steroid therapy; P = 0.08). However, the 18F-FDG uptake index in 30 LV segments with increased 18F-FDG uptake before steroid therapy was significantly decreased after steroid therapy (1.8 ± 0.6 before steroid therapy vs. 1.2 ± 0.2 after steroid therapy; P < 0.0001).
Two representative cases are shown in Figures 1 and 2.
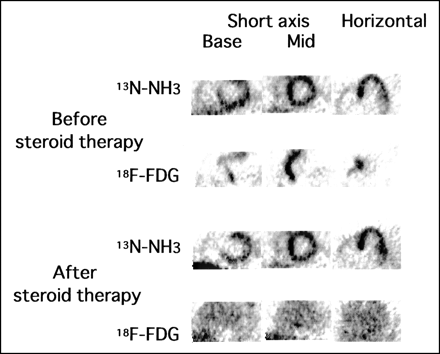
13N-NH3 and 18F-FDG PET images before and after steroid therapy in patient 12. Cardiac 13N-NH3 PET revealed moderate defects in basal anteroseptal wall of LV, and 18F-FDG PET revealed increased 18F-FDG uptake in basal anteroseptal-lateral wall and midanteroseptal wall of LV and free wall of right ventricle. After 1 mo of steroid therapy (prednisolone, 30 mg/d), increased 18F-FDG uptake in basal anteroseptal-lateral wall and midanteroseptal wall of LV was markedly diminished both in size and in intensity and that in free wall of right ventricle disappeared completely, whereas 13N-NH3 defects exhibited no significant change. 18F-FDG uptake indices of midanterior wall before and after steroid therapy were 2.8 and 1.8, respectively. Base = basal level of LV; Mid = middle level of LV.
13N-NH3 and 18F-FDG PET images before and after steroid therapy in patient 15. Cardiac 13N-NH3 PET revealed moderate defects in basal ventricular septum, and 18F-FDG PET revealed increased 18F-FDG uptake in basal and midanteroseptal wall of LV and free wall of right ventricle. After 1 mo of steroid therapy (prednisolone, 30 mg/d), increased 18F-FDG uptake in basal and midanteroseptal wall of LV and free wall of right ventricle disappeared completely, whereas 13N-NH3 defects exhibited no significant change. 18F-FDG uptake indices of midseptum before and after steroid therapy were 3.0 and 1.2, respectively. Base = basal level of LV; Mid = middle level of LV.
DISCUSSION
13N-NH3 defects and increased 18F-FDG uptake on cardiac 13N-NH3/18F-FDG PET were observed frequently in patients with cardiac sarcoidosis diagnosed with orthodox diagnostic criteria for cardiac sarcoidosis. 13N-NH3 defects were observed frequently in the basal anteroseptal wall of the LV, and increased 18F-FDG uptake was observed frequently in the basal and midanteroseptal-lateral wall of the LV. Increased myocardial 18F-FDG uptake was observed even in patients with no abnormal myocardial accumulation of 67Ga. Moreover, the increased myocardial 18F-FDG uptake was significantly diminished both in size and in intensity after steroid therapy. Thus, our study suggests the possibility of increased 18F-FDG uptake in the heart as a sensitive marker of cardiac sarcoidosis disease activity.
Scintigraphy with 67Ga has been used to diagnose and assess disease activity of sarcoidosis (6,14–19). The mechanism of 67Ga uptake is unclear in many disorders in which this tracer is used for diagnostic purpose. However, it is believed that 67Ga is actively taken up by macrophages in the lesions in patients with active sarcoidosis. Measurable uptake of 67Ga is interpreted as evidence of active inflammatory disease. Pulmonary uptake of 67Ga was observed in >90% of active cases of pulmonary sarcoidosis (20) and was markedly decreased after steroid therapy (21). However, little information concerning diagnosis of cardiac sarcoidosis with 67Ga scintigraphy has been available. Okayama et al. investigated 2 patients with myocardial accumulation of 67Ga and concluded that patients with myocardial uptake of 67Ga might be more responsive to steroid therapy (5).
Because only 3 of 15 patients with cardiac sarcoidosis, who underwent 67Ga scanning, exhibited myocardial 67Ga accumulation in our study, myocardial 67Ga scintigraphy was considered an insensitive method for detection of cardiac involvement of sarcoidosis. However, because we did not perform myocardial 67Ga SPECT on all patients in this study, which would be expected to increase the sensitivity of detection of myocardial accumulation of 67Ga, myocardial 67Ga SPECT must be performed in all patients with cardiac sarcoidosis to evaluate the diagnostic accuracy of myocardial 67Ga scintigraphy.
Decreased 201Tl uptake of the myocardium in patients with cardiac sarcoidosis is considered fibrogranulomatous replacement of the myocardium (1,2,5). In the remission stage of cardiac sarcoidosis, myocardium is replaced predominantly by fibrous tissue rather than by granulomatous tissue with inflammatory cell infiltration. Because myocardial 201Tl scintigraphy detects cardiac sarcoidosis with and without active inflammation, it may be a sensitive but nonspecific method for detection of active cardiac sarcoidosis (5). Likewise, 13N-NH3 defects might represent fibrogranulomatous replacement of myocardium with and without active inflammation and might be sensitive, but nonspecific, for detection of active cardiac sarcoidosis because 13N-NH3 is a perfusion tracer like 201Tl. In 2 patients (patients 9 and 17) in our study, 201Tl SPECT could not identify any perfusion defect, whereas 13N-NH3 PET identified a perfusion defect in the basal septum. Because PET has a higher spatial resolution than SPECT, 13N-NH3 PET has the potential to detect a perfusion abnormality more sensitively than 201Tl SPECT.
Recent studies found that 18F-FDG accumulated in the lung and bilateral hilar lymph nodes in patients with sarcoidosis (10,11). The cellular uptake of 18F-FDG in sarcoidosis is related to inflammatory cell infiltrates, which are composed of lymphocytes, macrophages, and epithelioid cells from monocytes, because 18F-FDG has been observed in vitro to be accumulated by leukocytes (22), lymphocytes, and macrophages (23). In patients with pulmonary sarcoidosis, 18F-FDG uptake of lung was concordant with histologic activity of pulmonary sarcoidosis, and the 18F-FDG uptake of lung was decreased after high-dose steroid therapy (10). Thus, 18F-FDG PET might provide a means of assessment of disease activity of pulmonary sarcoidosis.
Although no study investigating the usefulness of myocardial 18F-FDG PET for diagnosis of myocardial involvement of sarcoidosis has been available, 18F-FDG might accumulate in the myocardium of patients with cardiac sarcoidosis, as it does in pulmonary sarcoidosis. In patients with cardiac sarcoidosis, 18F-FDG uptake by the heart might be concordant with inflammation activity of cardiac sarcoidosis. This consideration is consistent with the findings of diminished 18F-FDG uptake in the myocardium after steroid therapy in our patients with cardiac sarcoidosis. Increased myocardial 18F-FDG uptake was observed in 10 patients without abnormal myocardial accumulation of 67Ga. Therefore, 18F-FDG PET has the potential to detect myocardial involvement of sarcoidosis with active inflammation more sensitively than 67Ga scanning. Takeda et al. (12) recently reported a case of cardiac sarcoidosis with third-degree atrioventricular block. In this case, PET revealed decrease 13N-NH3 uptake and strongly increased 18F-FDG uptake in the basal septal segment. Moreover, both findings disappeared and complete atrioventricular block improved to first-degree atrioventricular block after steroid therapy. These findings were concordant with our results.
In our study, 13N-NH3 defects were observed frequently in the basal anteroseptal wall of the LV, and increased 18F-FDG uptake was observed frequently in the basal and midanteroseptal-lateral wall of the LV. Both 13N-NH3 defects and increased 18F-FDG uptake were rare in the apex. These findings were concordant with previous pathologic and morphologic studies (24–26) indicating that cardiac involvement of sarcoidosis was common in the basal portion of the ventricular septum and free wall with sparing of the apex. Because 13N-NH3 defects or increased 18F-FDG uptake localized to such portions is uncommon in coronary artery disease, 13N-NH3 defects in the basal anteroseptal wall of the LV or increased 18F-FDG uptake in the basal and midanteroseptal-lateral wall of the LV might be specific for cardiac sarcoidosis. Moreover, in our study, 5 patients showed significant 18F-FDG uptake in hilar or mediastinal lymph nodes. Increased 18F-FDG uptake in the heart accompanied with significant 18F-FDG uptake in hilar or mediastinal lymph nodes might be specific for cardiac sarcoidosis.
In our study, 2 patients (patients 1 and 2) diagnosed as having cardiac sarcoidosis with orthodox diagnostic criteria exhibited no abnormal findings on PET. These patients exhibited only frequent premature ventricular contractions as a clinical manifestation of cardiac involvement of sarcoidosis. Because sarcoidosis lesions were not revealed in their hearts histologically, their premature ventricular contractions might have resulted from causes other than cardiac sarcoidosis.
The major limitation of this study was the small patient population. To confirm our results, studies should be performed in a larger population prospectively.
Our results demonstrated that increased 18F-FDG uptake was a sensitive indicator of cardiac involvement of sarcoidosis. However, we did not investigate the specificity or positive predictive value of 13N-NH3/18F-FDG PET for diagnosis of cardiac sarcoidosis. A previous study (27) found that some patients with idiopathic dilated cardiomyopathy exhibited heterogeneous myocardial glucose uptake, which was related to poor prognosis and lack of improvement of LV function after medical treatment. A more recent report (28) revealed that 6%–40% of myocardial segments in idiopathic dilated cardiomyopathy exhibited increased 18F-FDG uptake. To confirm the diagnostic accuracy of increased 18F-FDG uptake for diagnosis of cardiac sarcoidosis, the prevalence of increased 18F-FDG uptake in patients with sarcoidosis but no cardiac involvement or in patients with heart disease other than cardiac sarcoidosis must be investigated.
In this study, we defined a myocardial segment with normal 13N-NH3 uptake and minimal % 18F-FDG as a normal control segment. However, we did not confirm whether such myocardial segments were truly normal. In an advanced stage of cardiac sarcoidosis, there might be no normal myocardium remaining. Although the measurement of absolute regional myocardial glucose utilization might be preferable for detection of involved myocardium, this is complicated and inapplicable to 18F-FDG SPECT.
CONCLUSION
In this study, we investigated whether 13N-NH3/18F-FDG PET could identify cardiac involvement in patients with sarcoidosis diagnosed with orthodox diagnostic criteria for cardiac sarcoidosis. 13N-NH3 defects and increased 18F-FDG uptake on cardiac 13N-NH3/18F-FDG PET was observed frequently, and the increased myocardial 18F-FDG uptake was diminished in size and in intensity after steroid therapy. Our findings suggest that cardiac 13N-NH3/18F-FDG PET is the most useful both for identification of cardiac involvement of sarcoidosis and for assessment of cardiac sarcoidosis disease activity.
Footnotes
Received Oct. 25, 2002; revision accepted Mar. 10, 2003.
For correspondence or reprints contact: Hiroyuki Yamagishi, MD, Department of Internal Medicine and Cardiology, Osaka City University Graduate School of Medicine, 1-4-3 Asahi-Machi, Abeno-Ku, Osaka, 545-8585, Japan.
E-mail: yamagishi{at}med.osaka-cu.ac.jp