Abstract
The purpose of this study was to verify the feasibility and usefulness of a new SPECT method, called triple injection of 99mTc-ethylcysteinate dimer (TIE), in evaluation of the delayed or poor appearance of acetazolamide (ACZ) effects in patients with chronic cerebral ischemic disease. Methods: Three equal-volume splits of 99mTc-ethylcysteinate dimer were intravenously administered, and 1,000 mg ACZ were used as a vasodilator. A middle cerebral artery territory in the lateral ventricle was used as a region of interest. The data at rest and at 7.5 and 20 min after ACZ challenge (ACZ 7.5 and ACZ 20, respectively) were obtained by dynamic SPECT, and a time response curve to ACZ was obtained through the relative ratio of regional counts to the data at rest, not through regional cerebral blood flow. Nine cases of complete occlusion of the internal carotid artery (IC) and 6 cases of severe IC stenosis were analyzed. Results: In 12 healthy volunteers (24 cerebral hemispheres) using a placebo (negative control), the values at rest and at rest 7.5 and rest 20 (corresponding to ACZ 7.5 and ACZ 20, respectively) were 100%, 100.4% ± 2.8%, and 99.6% ± 3.6%, respectively, indicating the accuracy of the TIE method. In a positive control using 24 normal cerebral hemispheres, prompt maximal vasoreactivity at ACZ 7.5 (124.5% ± 8.0%) was confirmed, as was continuous vasoreactivity until ACZ 20 (130.1% ± 12.8%). The values between ACZ 7.5 and ACZ 20 were not statistically different. Patients with complete IC occlusion exhibited a poor response at ACZ 7.5 despite a normal response at ACZ 20 (delayed response). Furthermore, in patients with severe IC stenosis, restoration of cerebrovascular reactivity after carotid endarterectomy was confirmed not only at ACZ 20 but also at ACZ 7.5. Conclusion: The TIE method using SPECT may be a potentially useful and sensitive strategy in clinical evaluation of the delayed or poor appearance of ACZ effects in patients with chronic cerebrovascular ischemic disease.
Acetazolamide (ACZ) is well known as a potent vasodilator in the cerebral vessels and is frequently used in assessing the cerebral perfusion reserve (vasoreactivity). ACZ is known to increase blood flow rapidly within 2 min after intravenous administration, reaching a maximum 10 to 25 min later (1–4). Kuwabara et al. (5) reported that a PET study showed ACZ effects to reach a maximum within 5 min after intravenous administration on the nonoccluded side. The mechanism by which the regional cerebral blood flow (rCBF) increases after ACZ challenge is believed to be inhibition of carbonic anhydrase in the erythrocytes (1,2,4). Clinically, many studies have shown that patients with reduced cerebral vasoreactivity to ACZ have an increased risk of ischemic attack (6–8). ACZ is frequently used in assessing surgical indications for carotid endarterectomy (CEA) or external carotid–internal carotid artery (IC) bypass surgery in cases with IC stenosis or occlusion (9–11). Regarding so-called split-dose injections, Hattori et al. (12) previously reported a 1-d protocol with a double injection of 99mTc-ethylcysteinate dimer (ECD) for the assessment of cerebral perfusion reserve with ACZ. However, the time at which rCBF is measured after ACZ challenge varies between 5 and 20 min. Kuwabara et al. (5) reported that in cases of chronic ischemic cerebral disease, a PET study showed a significant difference in rCBF increase between 5 and 20 min after ACZ challenge on the occluded side (delayed response). The study also showed the steal phenomenon at 5 min after ACZ challenge. These results suggest the importance of establishing a suitable time for measurement of rCBF.
SPECT is more widely used than PET to assess rCBF and cerebral reserve because the cost and technical complexity of PET limit its clinical availability. In this study, a new SPECT method, triple injection of ECD (TIE), was used to assess the time dependency of vasoreactivity to ACZ. We have already reported our preliminary methodology, but not clinical data, for TIE (13). Our goal was to further verify the feasibility and usefulness of the TIE method in evaluation of the delayed or poor appearance of ACZ effects in patients with chronic cerebral ischemic disease.
MATERIALS AND METHODS
Subjects
For a negative control, saline instead of ACZ was intravenously injected into 12 healthy volunteers (24 cerebral hemispheres). For a positive control, 12 healthy volunteers (24 cerebral hemispheres) were used. Vasoreactivity was examined in 9 patients with complete IC occlusion; both the occluded and the nonoccluded sides were analyzed. Surgical effects on vascular reactivity were examined in 6 patients with cervical carotid artery stenosis. No hyperventilation occurred during ACZ loading, and no patients with lung dysfunction were examined. The degree of cervical carotid artery stenosis was evaluated using the criteria of the European Carotid Surgery Trial (14). We defined the degree of cervical carotid artery stenosis as follows: mild was 0%–29%, moderate was 30%–69%, and severe was 70%–99%.
SPECT Acquisition and Data Reconstruction
The protocol of the TIE method is schematically illustrated in Figure 1. As a tracer, 999 MBq 99mTc-ECD were accurately divided into 3 parts, with 333 MBq intravenously injected each time. The first injection of 99mTc-ECD was administered, and radionuclide angiography was performed for 2 min so that the method would be noninvasive, like the Patlak plot (15,16) and the resting and vascular reserve (17) methods. The only rest data were quantitatively obtained by the previously described brain uptake ratio method to evaluate the TIE data on clinical course (18). Briefly, brain uptake ratio is a new index of cerebral blood flow and is calculated by dividing the brain counts in the anterior planar image (60–80 s after injection of 99mTc-ECD) by the summation of the counts in the aortic arch during first transit of radionuclide. The brain uptake ratio correlated well with the brain perfusion index obtained with the Patlak plot method (18) (r = 0.96; P < 0.001). After 5 min, 1,000 mg of ACZ as a vascular dilator were intravenously injected, and the rest data were then collected by dynamic SPECT. Next, a second 99mTc-ECD (333 MBq) increment was injected 7.5 min after the ACZ challenge, and the data (ACZ 7.5) were obtained. ACZ 7.5 data were calculated by subtraction of the rest data, because of the accumulation of the first and second injections of 99mTc-ECD. Then, 20 min after the ACZ challenge, a third 99mTc-ECD (333 MBq) increment was injected, and the data (ACZ 20) were obtained. ACZ 20 data were also calculated by subtraction of the rest and ACZ 7.5 data. The time response curve to ACZ was shown as a relative ratio of regional counts under the condition that the rest data (of the right side) were 100%. A middle cerebral artery territory in the lateral ventricle (13) was used as a region of interest. The Student t test was used for statistical analysis.
Schematic illustration of SPECT method involving triple injection of ECD (TIE).
Serial dynamic SPECT scans (2.5 min × 13) were obtained with a GCA-7200A/DI gamma camera (Toshiba, Tokyo, Japan) equipped with low-energy general-purpose collimators and continuous mode. All data were prefiltered with a Butterworth filter (cutoff frequency, 0.24 cycle per pixel; order, 4) followed by reconstruction using a ramp filter and display on a 64 × 64 matrix. The pixel size was 4.7 × 4.7 mm, and the slice thickness was 9.4 mm. Furthermore, accumulation was mathematically adjusted for the natural decay of radioactivity. Neither attenuation correction nor scatter correction was performed.
RESULTS
Negative Control
As a negative control, 12 healthy volunteers (24 cerebral hemispheres) were administered saline instead of ACZ, and the values at rest (before ACZ), rest 7.5 (corresponding to ACZ 7.5), and rest 20 (corresponding to ACZ 20) were determined. If the value at rest was 100%, the values at ACZ 7.5 and ACZ 20 were 100.4% ± 2.8% and 99.6% ± 3.6%, respectively (Fig. 2). These data imply the accuracy of the TIE method.
Time response curve for TIE method used on negative control, which was administered saline instead of ACZ. Placebo control error was calculated to be as much as 7.2%.
Positive Control
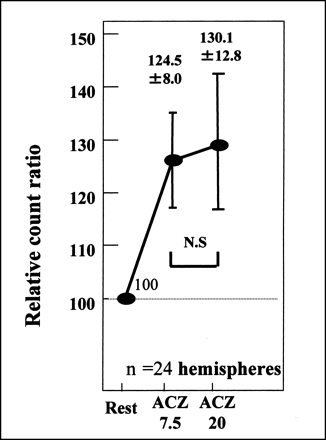
In 12 healthy volunteers (24 cerebral hemispheres), the TIE method indicated that rCBF responded promptly at ACZ 7.5 (124.5% ± 8.0%). This increase in rCBF continued for 20 min (ACZ 20) (130.1% ± 12.8%) (Fig. 3). The values between ACZ 7.5 and ACZ 20 were not statistically different.
Time response curve for TIE method used on positive control showed prompt response to ACZ at ACZ 7.5 and continuation of increased blood flow for 20 min (ACZ 20) (normal response). Differences between ACZ 7.5 and ACZ 20 values were not statistically significant (N.S).
Cases of Compete Occlusion of Carotid Artery
Of 9 cases of complete occlusion of the carotid artery, we chose 2 as representative (Table 1). In the first, a 51-y-old woman suddenly sustained motor aphasia and right motor weakness. CT and MRI showed a hemorrhagic infarction in the left temporal lobe. An intraarterial digital subtraction angiogram (IA-DSA) showed complete occlusion at the petrous portion of the left IC, and the rCBF in the left hemisphere was well compensated through the anterior communicating artery (ACOM), posterior communicating artery, and left ophthalmic artery. TIE analysis 1 y after onset showed that, compared with the nonoccluded side, rCBF on the occluded side was low at rest, showed no response at ACZ 7.5, and showed a good response at ACZ 20 (delayed response). Compared with the occluded side, the nonoccluded side also showed a delayed response pattern, with a high rCBF at rest (Fig. 4A). In the second representative case, a 73-y-old man suddenly sustained right motor weakness and dysarthria. MRI showed an embolic infarction in the left frontal lobe. IA-DSA showed complete occlusion of the left IC with good collateral blood flow through the ACOM and left ophthalmic artery. TIE analysis 4 mo after onset showed that the occluded side had a delayed response pattern. The nonoccluded side, by contrast, had a near-normal response as shown through comparison with the positive control (Fig. 4B).
Time response curves for TIE method used on cases of complete occlusion of carotid artery. Sides with complete occlusion responded poorly at ACZ 7.5 despite normal response at ACZ 20 (delayed response). Sides without occlusion showed 2 patterns of vasoreactivity (delayed or near-normal). (A) In case 1, delayed response occurred on both sides. (B) In case 2, near-normal response occurred on nonoccluded side. (C) Overall, delayed response occurred on occluded side, and differences between ACZ 7.5 and ACZ 20 values were statistically significant (P < 0.001). Near-normal response occurred for 5 of 9 nonoccluded sides, and differences between ACZ 7.5 and ACZ 20 values were statistically significant (P < 0.05). This near-normal response was significantly different at ACZ 7.5 (*), but not at ACZ 20 (**), from normal response in positive controls (P < 0.05).
ACZ Response Patterns to IC Occlusion
Overall, in 9 cases of complete occlusion of the carotid artery, TIE analysis showed a delayed response on all occluded sides, that is, a poor response to ACZ at ACZ 7.5 (102.1% ± 1.5%) and a good response at ACZ 20 (117.1% ± 4.9%). The values between ACZ 7.5 and ACZ 20 were statistically different (P < 0.001) (Fig. 4C). In patient 8, the occluded side showed the steal phenomenon at ACZ 7.5 and collateral flow was incomplete (Table 1). On nonoccluded sides, 2 response patterns were seen, a delayed response in 4 patients and a near-normal response in 5 patients (Table 1). For the near-normal responses, the values at ACZ 7.5 and ACZ 20 were 116.8% ± 6.5% and 124.2% ± 10.7%, respectively. The values between ACZ 7.5 and ACZ 20 for near-normal responses were statistically different (P < 0.05). Near-normal responses were also statistically different at ACZ 7.5, but not at ACZ 20, from normal responses in the positive control (P < 0.05) (Fig. 4C).
Cases of Stenosis of Carotid Artery
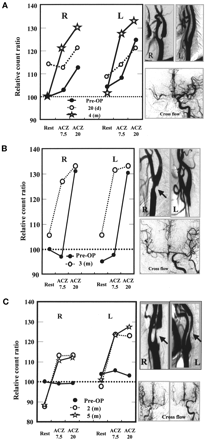
Of 6 cases of stenosis of the carotid artery (CEA cases), we chose 3 as representative (Table 2). In the first, a 74-y-old man suddenly sustained left motor weakness; MRI showed a small lacunar infarction in the right centrum semiovale. IA-DSA showed severe stenosis in the right cervical IC and no stenosis in the left. Collateral flow through the ACOM was good. Several months later, CEA was performed on the right side. Preoperatively, both sides exhibited a delayed response pattern. Twenty days after the operation, both sides still showed a delayed response pattern, but rCBF at rest was higher than it had been preoperatively on both sides. Four months later, a normal response pattern was restored on both sides (Fig. 5A). In the second representative case, a 68-y-old man was incidentally shown to have moderate IC stenosis on the right side. The left side had no IC stenosis. Preoperatively, both sides had a delayed response pattern. Collateral flow through the ACOM was good. Three months after the operation, the vasoreactivity at ACZ 7.5 was restored on both sides (normal response pattern) (Fig. 5B). In the third case, a 74-y-old man suddenly experienced dysarthria and left sensory disturbance. MRI showed a small lacunar infarction in the right centrum semiovale. IA-DSA showed severe IC stenosis bilaterally, but more so on the right side. Collateral flow through the ACOM was good on both sides. CEA was performed on the right side. Preoperatively, TIE analysis showed no response pattern on either side. The normal response pattern was restored on both sides 2 mo after the operation and was also confirmed at 5 mo (Fig. 5C).
Changes in vasoreactivity for TIE method after CEA. (A) In case 1, 20 d after CEA (○), response was still delayed on both sides. Four months after CEA (☆), vasoreactivity normalized. (B) In case 2, 3 mo after CEA (○), vasoreactivity at ACZ 7.5 was remarkably restored (normalization). (C) In case 3, vasoreactivity became normal 2 mo after CEA (○).
Summary of Cases of Carotid Artery Stenosis
Overall, in 6 patients with moderate to severe stenosis of the cervical carotid arteries, TIE analysis was undertaken before and after endarterectomy (Table 2). In 2 of the patients (patients 1 and 2), both sides had a delayed response pattern preoperatively even though IC stenosis was unilateral. In 2 other patients (patients 4 and 5) with severe unilateral IC stenosis, both sides had a normal response pattern. In patients with normal preoperative to normal postoperative responsive patterns, rCBF at rest increased postoperatively. CEA brought about a normal response pattern in all patients with IC stenosis.
DISCUSSION
In this study, we verified the feasibility and usefulness of a new SPECT method, TIE, in evaluation of the delayed or poor appearance of ACZ effects in patients with chronic cerebral ischemic disease. Among the radioactive tracers, 99mTc-ECD was chosen on the following basis. In general, 99mTc-ECD is characterized by prompt uptake from the bloodstream into brain tissues through the blood–brain barrier. 99mTc-ECD is also quite stable (19–21). 99mTc-ECD washout from brain tissues is minimal during the first 50 min after intravenous administration and is not affected by ACZ (12). 99mTc-ECD accumulation in brain tissues depends on esterase activity and reflects not only cerebral blood flow but also cerebral metabolism (22). Studies have shown that ECD SPECT is likely able to accurately reflect regional blood flow in normal and pathologic states (23) and that uptake of 99mTc-ECD correlates significantly with rCBF (24). 99mTc-ECD produces images of excellent quality because background accumulation is low (22). In this study, a modification of the resting and vascular reserve method (17) was applied to produce 3 equal-volume splits of 99mTc-ECD for injection and to make the influence of subtraction of 99mTc-ECD accumulation negligible.
Validation of the TIE method requires 3 conditions. First, placebo control (negative control) data without ACZ must show near-equality between values at rest (before ACZ), at rest 7.5 (corresponding to ACZ 7.5), and at rest 20 (corresponding to ACZ 20). Second, a positive control using normal brains with ACZ must show prompt maximal vasoreactivity at ACZ 7.5. Third, continuous vasoreactivity until ACZ 20 must exist. In this study, the placebo control error was calculated to be as much as 7.2%. The positive control confirmed that prompt maximal vasoreactivity at ACZ 7.5 occurred, as did continuous vasoreactivity until ACZ 20. These findings show the feasibility of the TIE method. In general, the lack of linearity at a high rCBF with 99mTc-ECD is known (25). In this study, however, the authors evaluated ACZ vasoreactivity only by time response curve patterns (relative count ratio), not by rCBF values. Therefore, the lack of linearity, especially at ACZ 20, might occur in this study.
In all cases of complete occlusion of the carotid artery, TIE showed rCBF to have a delayed response pattern to ACZ (poor at ACZ 7.5 and good at ACZ 20) on the occluded sides. Therefore, a split-dose injection analysis would have shown good response patterns in all cases because of the absence of ACZ 7.5 data. In patient 2, with carotid artery stenosis, vasoreactivity was remarkably restored at ACZ 7.5 but was unchanged at ACZ 20 after CEA. Therefore, a split-dose injection analysis would have shown no vasoreactive effect after CEA. These findings indicate that the TIE method, in comparison with a split-dose injection, may be sensitive to the assessment of vasoreactivity in cerebrovascular ischemic disease.
Vasoreactivity, not only ipsilaterally but also contralaterally, returned to normal postoperatively in 3 cases of IC stenosis with abnormal vasoreactive patterns (patients 1–3). We speculate that in cases of unilateral stenosis (patients 1 and 2), rCBF on the stenosed side was compensated for by the nonstenosed side through the ACOM. In addition, prior vasodilation occurred on the nonstenosed side. Therefore, further vasodilation after ACZ challenge developed poorly (delayed response) not only on the stenosed side but also on the nonstenosed side. We speculate that no compensation of rCBF was required after CEA and that vasoreactivity to ACZ was normalized on both sides. In patient 3, with bilateral severe IC stenosis, we speculate that maximum vasodilation occurred on both sides and that no further vasodilation developed after ACZ challenge. We speculate that, after CEA, rCBF on the nonoperated side was compensated for through the ACOM from the operated side. Vasoreactivity was then remarkably restored on both sides. Therefore, an additional CEA may not be required on the contralateral side in some cases of severe bilateral IC stenosis. In some cases reported in the literature, vasoreactivity after CEA was improved not only ipsilaterally but also contralaterally (9,26). Cikrit et al. (9) reported that, in 12 of 51 patients with unilateral severe stenosis of the cervical IC, vascular reactivity also improved contralaterally. This improvement was thought to be caused by recovery of dysautoregulation on both sides.
Recently, perfusion MRI has been widely used to evaluate acute cerebrovascular ischemic disease. Perfusion MRI has high resolution in space and time but is limited in the assessment of cerebrovascular reserve and in quantitative analysis (27). Consequently, SPECT analysis is still required to clinically evaluate cerebrovascular reserve. Future consideration should be given to measurement points in addition to 7.5 and 20 min and, depending on clinical conditions, to other brain regions of interest, such as vascular territories.
CONCLUSION
The TIE method using SPECT may be a potentially useful and sensitive strategy in the clinical evaluation of cerebrovascular reserve in patients with chronic cerebrovascular ischemic disease.
Acknowledgments
The authors sincerely thank Dr. Joji Urata, Yoshihiko Mizuta, Kazuhiro Okada, and Rie Takaki for valuable discussions and Clayton Young (University of Toronto, Toronto, Ontario, Canada) for editorial assistance.
Footnotes
Received Jun. 22, 2001; revision accepted Jan. 16, 2002.
For correspondence or reprints contact: Masaji Murakami, MD, Department of Neurosurgery, Kumamoto Takumadai Hospital, Onoue 1-14-27, Kumamoto 862-0913 Japan.
E-mail: mmurakami{at}po.infobears.ne.jp