Abstract
It is not known whether cellular metabolic disorders play a role in the decreased tracer uptake that is documented by conventional SPECT during low-flow ischemia or stunning. This study sought to determine the impact of low-flow ischemia and stunning on the kinetics of 201Tl and MIBI across the plasma membrane of myocytes. Methods: The global myocardial retention (Rf) of 201Tl and MIBI was determined in isolated working hearts from rabbits, perfused with red blood cell–enhanced solution. Experiments were performed in normoxia, with physiological values of coronary flow (N; n = 16); in low-flow ischemia, with a >50% reduction of coronary flow and a ≥ 20-mm Hg fall in systolic left ventricle pressure (L; n = 15); and in stunning, with 15 min of acute ischemia followed by reperfusion (S; n = 15). Concentration ratios across the plasma membrane of myocytes were also determined for both tracers and expressed as Ci/Cc, where Ci is interstitial activity determined with microdialysis, and Cc is activity from cellular space determined from Rf and Ci values. Results: There was a slight increase in average values of Ci/Cc in ischemia, but not in stunning, for 201Tl (L, 0.011 ± 0.006 vs. N, 0.006 ± 0.004 [P < 0.05]; S, 0.007 ± 0.004 vs. N [not significant]) and for MIBI (L, 0.011 ± 0.008 vs. N, 0.005 ± 0.004 [P < 0.05]; S, 0.005 ± 0.003 vs. N [not significant]). Moreover, ischemia and stunning had no deleterious effects on the average values of global myocardial retention for 201Tl (L, 0.63 ± 0.09 vs. N, 0.50 ± 0.14 [P < 0.05]; S, 0.59 ± 0.10 vs. N [P < 0.05]) or for MIBI (L, 0.45 ± 0.10 vs. N, 0.31 ± 0.09 [P < 0.05]; S, 0.41 ± 0.12 vs. N [P < 0.05]). In fact, these values were significantly enhanced in the 2 situations. Conclusion: The kinetics of 201Tl and MIBI across the plasma membrane of myocytes were affected only poorly by low-flow ischemia and not at all by stunning, without any deleterious effects on myocardial retention of both tracers. During low-flow ischemia or stunning, therefore, the information provided by 201Tl or MIBI SPECT is expected to depend on myocardial perfusion but not on cellular metabolic disorders.
By analyzing the myocardial uptake of certain radiotracers, SPECT allows the detection and localization of abnormalities in myocardial perfusion. However, SPECT images might also be influenced, during ischemia, by metabolic factors that interact with tracer uptake by myocytes (1–12).
The impact of ischemia on the myocardial kinetics of 201Tl and MIBI remains controversial. In isolated heart models, it has been shown that the reduction of coronary flow is followed by an increase in tracer extraction and retention by the entire myocardial tissue (13,14); whereas, on myocyte cultures, it has been shown that certain metabolic disorders that may be associated with ischemia (cellular anoxia, acidosis) can decrease the cellular uptake of 201Tl and MIBI (7,8).
Currently, it is not known whether cellular metabolic disorders play a significant role in the decreased tracer uptake documented by SPECT during low-flow ischemia or stunning. In both clinical situations, it is unclear whether the information provided by 201Tl or MIBI SPECT depends on myocardial perfusion, cellular metabolic disorders, or both. The answer to this question could have important consequences for the interpretation of SPECT images. Previously, the transit of tracers through the plasma membrane of myocytes had only been analyzed in cell cultures (7,8). However, such models do not allow studies to be performed under ischemic conditions. In addition, they do not allow assessment of tracer kinetics within the entire myocardial tissue while taking into account tracer transit across the capillary membrane. This transit is, however, considerably variable depending on the tracer used and the perfusion flow (12,15,16).
Using an isolated heart model, we have applied an original technique of microdialysis that allows us to measure tracer activity from the interstitial space and estimate tracer activity from cellular space. In the current study, this model was used to analyze the impact of low-flow ischemia and stunning on the kinetics of 201Tl and MIBI between the interstitial and cellular spaces and, thus, across the plasma membrane of myocytes.
MATERIALS AND METHODS
Experimental Model
The isolated heart model perfused with red blood cell–enhanced perfusate has been described in detail previously (15). During the experiments, the perfusate was maintained at 37°C and oxygenated with a gas mixture containing 20% O2, 5% CO2, and 75% N2. Hearts from New Zealand White rabbits (2.0–2.5 kg) were excised and fixed to the blood-perfusion system, and the discharge of the perfusion pump was regulated so that perfusion pressure ranged from 75 to 80 mm Hg, corresponding to the diastolic aortic pressure for rabbits as determined in vivo (17).
The microdialysis probe was inserted in the left ventricle (LV) using a small introducer (which was subsequently withdrawn) that allowed placement of the dialysis membrane within the LV wall. As previously described, the probe was linear and flexible and consisted of a 12-mm polyacrilonitrile membrane (AN69 Hospal, Lyon, France; 50-kDa cutoff) connected to 2 silica capillary tubes (Phymep, Paris, France; external diameter, 150 μm; internal diameter, 75 μm) fixed at each end of the dialysis membrane (18). During the experiments, the probe was perfused with a Ringer lactate solution (NaCl, 6 g · L−1; KCl, 0.4 g · L−1; CaOHCl, 0.27 g · L−1; Na+ lactate, 2.6 g · L−1) at a constant flow of 4 μL · min−1 (EP244 pump; Harvard Apparatus, Cambridge, MA).
Hearts developing less than 80 mm Hg systolic pressure during an initial 15-min equilibrium period were not considered for the study. At the end of this period, 3 different protocols of experiments were carried out. For normoxic experiments, coronary flow was unmodified throughout the rest of the experiment. For ischemic experiments, coronary flow was progressively decreased to less than 50% until a ≥20-mm Hg fall in systolic pressure occurred, and thereafter the coronary flow rate was kept constant. For stunning experiments, a 15-min period of acute ischemia was obtained by decreasing coronary flow to 10% of its initial value; thereafter, we attempted to bring it back to its initial value without exceeding a perfusion pressure of 100 mm Hg.
At the end of the experiments, the hearts were immediately weighed (Ww). Dry weight (Wd) was calculated after a 48-h desiccation in a 42°C thermostatic chamber, and the fraction of water content inside the myocardial tissue was calculated with the formula (Ww – Wd)/Ww.
Hemodynamic and Oximetric Parameters
Coronary perfusion pressure and systolic, diastolic, and mean LV pressures were continuously monitored. Coronary flow was measured through the coronary venous drainage and expressed in mL · min−1 · g−1 Wd. PO2, PCO2, pH, and hemoglobin saturation were measured in venous and arterial samples (ABL4 Radiometer, Copenhagen, Denmark). Oxygen extraction (cm3 · mL−1) and myocardial oxygen consumption (cm3 · min−1 · g−1 Wd) were then calculated using a standard formula (19).
Radiopharmaceutical Analysis
First-pass myocardial kinetics of 201T1 (CIS Bio International, Gif-sur-Yvette, France) and 99mTc-MIBI (Dupont de Nemours, les Ullis, France) were analyzed using 125I-labeled bovine serum albumin (NEN Life Science Products, Boston, MA) as a vascular reference tracer (14,15). The radiochemical purity of MIBI and 125I albumin was >95% in all cases.
A mixed isotope bolus containing 20–30 μCi of each of the 3 radiotracers was prepared in a total volume of 0.4 mL. Half of this volume was injected above the aortic canula after a 15-min equilibration period had been performed in each experimental condition (normoxia, ischemia, and stunning). The remaining half of the bolus solution was diluted in aliquots (n = 12) to calculate the respective proportion of each tracer in the injected bolus.
Immediately after bolus injection, a coronary venous sampling was started from the pulmonary artery drainage, as described previously (15). Three minutes after bolus injection, 4 consecutive dialysate samples were collected over 10-min periods. The dialysate tubes were weighed before and after collection (Mettler Toledo, Greifensee, Switzerland; 10–3 g precision) to allow precise determination of collected volumes. The initial 3-min interval was chosen to start the analysis just after the myocardial extraction of tracer. In preliminary experiments, indeed, activity from the isolated heart had been followed up with a gamma camera after injection of a vascular tracer (99mTc-labeled albumin), and in all cases at least 85% of the bolus was washed out from the myocardium 3 min after injection.
Activities from 201Tl, 99mTc, and 125I were measured in each of the venous samples, dialysate samples, and aliquots with an automated γ-counter (GAMMAmatic; Kontron Instruments, Basel, Switzerland) using a previously described method (13–15). For each venous sample, an instantaneous extraction fraction (E) at a given time (t) was computed relative to the vascular reference tracer (125I-albumin) for both 201Tl and MIBI (13–15).
Three parameters were used to analyze the first-pass kinetics of tracers:
The maximal instantaneous extraction fraction (Epeak) gives an estimate of tracer diffusion from blood to myocardial tissue; it was used to calculate the capillary permeability–surface area product (PScap) with the Crone equation:
 Eq. 1
F being the coronary flow rate in mL · g−1 Ww (20,21).
Eq. 1
F being the coronary flow rate in mL · g−1 Ww (20,21).
The retention fraction (Rf) is the net tissue uptake of tracer at a given time (t), and it was calculated with the following formula, t0 being the time at the beginning of venous sampling and x, a variable of integration (13–15):
 Eq. 2
Rf’s value, corresponding to the time when more than 90% of 125I-albumin activity had appeared in the venous effluent (end of the myocardial extraction of tracer) was called net extraction (Enet).
Eq. 2
Rf’s value, corresponding to the time when more than 90% of 125I-albumin activity had appeared in the venous effluent (end of the myocardial extraction of tracer) was called net extraction (Enet).
The fractional escape rate (Fe) represents the proportion of tracer that is cleared from the myocardium at a given time (t) (14,15):
 Eq. 3
Eq. 3
For each experiment and for each of the 2 diffusible tracers, mean values of Rf and Fe were also calculated over each of the 4 consecutive 10-min periods corresponding to dialysate collection periods, and for the total 40-min period of kinetics analysis.
Calculation of Microdialysis Probe Recovery for 201Tl and MIBI
To determine tracer activity within the interstitial space, the microdialysis probe recovery was calculated in vitro for 201Tl and MIBI, as previously described (22,23). Briefly, multiple microdialysis probes were perfused with a Ringer lactate solution at increasing flow values ranging from 1 to 8 μL · min−1 and immersed in a 37°C Ringer lactate solution containing 200 MBq of both 201Tl and 99mTc-MIBI. At each perfusion flow value, multiple dialysate collections of 10 min each were performed. For a given tracer and at a given flow, probe recovery was calculated as the fraction of activity measured per milliliter of dialysate relative to that measured per milliliter of analyzed medium.
On the basis of these calculations, a perfusion flow of 4 μL · min−1 was chosen for subsequent experiments. Indeed, this value allowed both a relatively short sampling time of 10 min (collected volumes are sufficient), and a probe recovery high enough (0.97 for 201Tl and 0.40 for MIBI) to insure the detection of significant changes in activity from dialysate samples.
Determination of Tracer Interstitial and Cellular Retention
For each of the 4 samples and for each of the 2 tracers, activity from interstitial space was expressed per gram of interstitial fluid and as a fraction of total injected activity. To estimate jointly the cellular retention of the 2 tracers, tracer activity from interstitial fluid was subtracted from the average value of the tracer’s global myocardial retention in each of the 4 periods of dialysate sampling. The results were expressed as a fraction of the tracers’ injected activity and per gram of cellular compartment. For this calculation, the weight of the interstitial compartment was considered to represent 25% of that of the myocardial tissue and the weight of cellular compartment to the remaining 75%. Distribution was determined in this experimental model using EDTA, which has a distribution volume limited to the extracellular space (9,24). Finally, tracer distribution between the interstitial and cellular spaces was calculated for the 4 periods of dialysate collection using the ratio Ci/Cc, where Ci is the activity from interstitium (per gram of interstitial fluid) and Cc is the activity from cellular space (per gram of cellular compartment).
Statistics
Quantitative parameters were expressed as mean ± SD and compared with nonparametric tests. Paired comparisons were performed with Wilcoxon tests, and multiple paired comparison procedures with Kurskall-Wallis tests. For all tests, a probability value of <0.05 was considered to indicate a significant difference.
RESULTS
Hemodynamic and Oximetric Parameters
Sixteen experiments were conducted in normoxia, 15 in ischemia, and 15 in stunning. Comparisons between these 3 groups for hemodynamic and oximetric parameters are presented in Table 1 and illustrated in Figure 1. Compared with normoxic experiments, those performed in ischemia had 2.2-fold lower values for coronary flow. They also had markedly lower values for coronary perfusion pressure and systolic and mean LV pressures. In spite of enhanced oxygen extraction, ischemic experiments also had much lower values for myocardial oxygen consumption.
Variations in systolic contraction pressure (A) and coronary flow rates (B) between onset and end of experiments conducted in normoxia (○; n = 16), low-flow ischemia (•; n = 15), and stunning ( ; n = 15).
; n = 15).
Hemodynamic and Oximetric Parameters in Experiments Performed in Normoxia, Low-Flow Ischemia, and Stunning
For experiments performed during stunning, systolic and mean LV pressures, as well as coronary flow and myocardial oxygen consumption, progressively increased during the period of tracer kinetics analysis; they were initially lower than in normoxic experiments but became equivalent 40 min later. Compared with ischemic experiments, those performed during stunning had constantly higher values for coronary flow and perfusion pressure and, at the end of the experiments, they also had higher values for LV pressure and myocardial oxygen consumption.
Finally, the fraction of tissue water content calculated at the end of the experiments was equivalent between normoxic (0.75 ± 0.04) and ischemic (0.77 ± 0.02) experiments, but was significantly higher in stunning experiments (0.79 ± 0.02; P = 0.0004 vs. normoxia, and P = 0.004 vs. ischemia).
Kinetics of Tracers Between Interstitial and Cellular Spaces
When the entire period of kinetics is considered, ischemia, but not stunning, led to a slight increase in averaged values of Ci/Cc for 201Tl (ischemia, 0.011 ± 0.006 vs. normoxia, 0.006 ± 0.004 [P < 0.05]; stunning, 0.007 ± 0.004 vs. normoxia [not significant]) and for MIBI (ischemia, 0.011 ± 0.008 vs. normoxia, 0.005 ± 0.004 [P < 0.05]; stunning, 0.005 ± 0.003 vs. normoxia [not significant]).
When the 4 consecutive periods of dialysate sampling were analyzed, however, there was a temporal decay for the interstitial activities of 201Tl and MIBI (Fig. 2), as well as the corresponding Ci/Cc values (Fig. 3). For each of the 2 tracers and during all 4 periods of analysis, activities from interstitial space were higher in ischemia than in normoxia and stunning (Fig. 2).
Comparison of activity in interstitial fluid for 201Tl (A) and MIBI (B) during experiments conducted in normoxia (□; n = 16), low-flow ischemia (▪; n = 15), and stunning (▨; n = 15). Activities expressed as a fraction of total injected activity per milliliter of interstitial fluid for each of 4 consecutive 10-min periods following myocardial extraction of tracer. *P < 0.05.
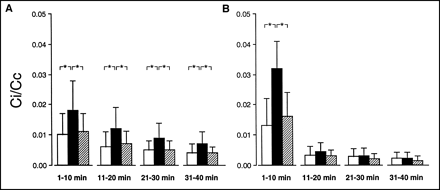
Comparison of tracer concentration ratios between interstitial (Ci [g−1]) and cellular (Cc [g−1]) spaces for 201Tl (A) and MIBI (B) during experiments conducted in normoxia (□; n = 16), low-flow ischemia (▪; n = 15), and stunning (▨; n = 15). Activity ratios determined for each of 4 consecutive 10-min periods following myocardial extraction of tracer. *P < 0.05.
For 201Tl, Ci/Cc was slightly but constantly higher in ischemia than in normoxia and stunning (Fig. 3). However, for MIBI, such differences in Ci/Cc were only observed in the initial 10 min, when the Ci/Cc values for MIBI were particularly high, and markedly higher than those for 201Tl.
Extraction and Retention of Tracers by Myocardial Tissue
Main results from the first-pass kinetics analysis of tracers are given in Table 2. All analyzed variables were significantly higher for 201Tl than for MIBI. For each of the 2 tracers, the capillary permeability–surface area product and the mean value of the fractional escape rate were lower in ischemia and stunning than in normoxia.
First-Pass Kinetics Parameters in Experiments Performed in Normoxia, Low-Flow Ischemia, and Stunning
During the entire period of kinetics analysis, there was a slight increase in the averaged values of the retention fraction in ischemia, but not in stunning, for 201Tl and MIBI (Table 2).
Figure 4 represents mean values of the myocardial retention fractions calculated for the 4 consecutive 10-min periods corresponding to the dialysate sampling. For each of the 2 tracers and during each of the 4 periods, these fractions were higher in ischemia and stunning than in normoxia.
Comparison of average tracer myocardial retention fractions for 201Tl (A) and MIBI (B) during experiments conducted in normoxia (□; n = 16), low-flow ischemia (▪; n = 15), and stunning (▨; n = 15). Fractions calculated for each of 4 consecutive 10-min periods following myocardial extraction of tracer. *P < 0.05.
DISCUSSION
Until now, only in vitro studies performed on myocyte cultures had allowed analysis of the distribution of 201Tl and MIBI between the extracellular and cellular spaces (7,8). In the current study, this distribution was directly determined within the myocardial tissue using an isolated rabbit heart model. For each of the 2 tracers, we found that the interstitial/cellular concentration ratios (Ci/Cc) were lower than 1% during normoxia and were therefore equivalent to the Ci/Cc ratios previously obtained for cell cultures (7).
In contrast to cell cultures, this model allowed us to analyze the impact of ischemia on extracellular/cellular ratios of tracers. Experiments were conducted during low-flow ischemia and also in the postischemic period during stunning, a situation that is frequently revealed by SPECT imaging (25). As already observed in several studies (26–28), both low-flow ischemia and stunning were associated with marked impairments in contraction pressure, myocardial oxygen consumption, and myocardial perfusion rates. For stunning, but not for low-flow ischemia, these abnormalities were spontaneously reversible (29), with a delayed return to normal after reperfusion.
For both 201Tl and MIBI, the average Ci/Cc ratios were only slightly increased during ischemia and were unchanged during stunning. This provides evidence that, within myocardial tissue, tracer kinetics across the plasma membrane were poorly altered by low-flow ischemia and not at all during stunning. However, when the time course of Ci/Cc was analyzed, we observed that the impact of ischemia was not the same for the 2 tracers.
For 201Tl, Ci/Cc was constantly higher in ischemia than in normoxia or stunning during the entire period of kinetics analysis. This observation is consistent with results obtained in cell cultures, where chemically induced cellular hypoxia was shown to have a marked inhibiting effect on 201Tl cellular uptake (8,30). Indeed, 201Tl crosses the plasma membrane of myocytes using mainly an active energy-consuming mechanism (Na/K pump) (31), which is affected by cellular hypoxia (7,8). However, when ischemia is followed by reperfusion, and in the absence of myocardial necrosis, it has been shown that there is a rapid return to normal of the transport mechanisms (9). This latter point likely explains why stunning had no significant impact on the Ci/Cc ratio of 201Tl.
In contrast to what was observed for 201Tl, the decrease during ischemia in the Ci/Cc ratio of MIBI was observed only during the first 10 min after the myocardial extraction of tracers. This could not be caused by a real impairment in the mechanism allowing this tracer to cross the plasma membrane of myocytes, because such impairment would have led to a sustained increase in extracellular/cellular concentration ratio (7). For MIBI, which is a cationic lipophilic molecule, transit through the plasma membrane is mainly mediated by membrane polarization (32). Our results are in accordance with those from cell culture studies, where this transit has been shown to be unaffected by cellular hypoxia until serious and presumably irreversible damage occurs (30).
The analysis of the first 10-min period following the myocardial extraction of tracer includes the initial phase of tracer transit from interstitial to cellular space. That is why interstitial concentrations of tracers were clearly higher initially than during subsequent periods. Also, during this initial period, it is not surprising to observe that Ci/Cc ratios for MIBI were higher than those for 201Tl. MIBI is, indeed, much more voluminous than the 201Tl atom, and its diffusion from extracellular to cellular space is markedly slower, a point that has already been documented in cell cultures (7).
In addition, it must be kept in mind that tracer interstitial transit also depends on the flow of interstitial fluid, along which the cellular substrates move toward cardiac cells. This role of interstitial flow is likely more important for molecules with high molecular weight, like MIBI, when the ability of such molecules to diffuse spontaneously toward cellular space is reduced (33).
The flow of interstitial fluid is mainly related to a hydrostatic pressure gradient located at the arterial pole of the capillary membrane; therefore, this flow is considerably impaired when capillary hydrostatic pressure decreases (33). In the current study, coronary hydrostatic pressure was decreased during low-flow ischemia during stunning. Thus, the decrease in interstitial fluid flow (related to the decrease in arterial hydrostatic pressure) could explain the slowing down during low-flow ischemia of MIBI transit from interstitial to cellular space. Whatever the mechanism, this original observation reveals that low-flow ischemia might also impair the interstitial transit of other compounds, such as pharmaceuticals or cellular substrates. Nevertheless, these limited kinetic changes across the plasma membranes of myocytes during low-flow ischemia had no deleterious effect on tracer retention within the entire myocardial tissue.
In contrast, both low-flow ischemia and stunning were observed to induce an increase in tracer initial extraction and a decrease in subsequent clearance rates across the capillary membrane, resulting in enhanced retention within the myocardial tissue for 201Tl and MIBI.
Myocardial clearance of 201Tl and MIBI is related to a retrodiffusion process through the capillary membrane. Clearance is, therefore, influenced by the tracer concentra-tion gradient across this membrane and also by its permeability and surface. Our results show that the decrease in this retrodiffusion seen during both low-flow ischemia and stunning is not related to lower levels of tracer interstitial concentration. However, it may be partly explained by the lower washout rate of tracer from the capillary space when coronary flow is reduced. In addition, our findings on the capillary permeability–surface area product show that stunning and, especially, low-flow ischemia were associated with marked impairment in tracer ability to diffuse across the capillary membrane. This phenomenon, which has already been documented in isolated heart preparations for both 201Tl and MIBI (9,13,15), might also play a role in the decrease of tracer clearance rates evidenced here during ischemia and stunning.
The increase in initial myocardial extraction of tracer also likely plays a major role in the enhanced myocardial retention documented here for 201Tl and MIBI during low-flow ischemia and possibly during stunning. Indeed, previous reports have already shown that this extraction depends strongly on coronary flow rates. More precisely, extraction increases when coronary flow decreases and, thus, when the capillary transit time of tracer is longer (13,15).
However, when extrapolating our observations to SPECT imaging, our study is limited by the particularities of the experimental model (isolated heart, animal species) and by the fact that our analysis was only performed at first-pass, neglecting the influence of tracer recirculation (13). Also, in clinical settings, the variable proportions of viable and nonviable cells within ischemic or stunned myocardial segments play an additional role in tracer uptake. This aspect cannot be considered in our model, in which all cells are supposed to be viable and in the same metabolic state.
Another limitation of this study is the fact that it was not possible to follow the volume of interstitial fluid along the experiments. In this study, the volume was considered to be stable and to represent 25% of that of the myocardial tissue (9,24). However, because myocardial edema can occur during reperfusion, the volume of the interstitial space increases progressively after sequences of ischemia–reperfusion (34). Our results concerning the fraction of water content inside the myocardium confirm the presence of myocardial edema at the end of stunning experiments. The main consequence is that the Ci/Cc ratios of tracers calculated during stunning were probably overestimated. However, this point does not affect our observation that stunning did not increase the Ci/Cc ratios of tracers and, thus, did not affect tracer kinetics across the plasma membrane of myocytes. In addition, the results of previous in vivo animal experiments have shown that MIBI SPECT defects observed in stunning were not attributable to any metabolic inhibition in tracer uptake (35,36).
Finally, our results suggest that in both low-flow ischemia and stunning, the information provided by 201Tl or MIBI SPECT is unrelated to any metabolic impairment in cellular uptake of tracer. In addition, because enhanced myocardial retention was observed in underperfused areas, the decrease in tracer uptake documented by SPECT in these areas is likely less than the corresponding decrease in perfusion flow, as shown in previous studies (4,15,26).
Nevertheless, it must be pointed out that the flow-dependence of myocardial uptake of 201Tl and MIBI is particularly useful for SPECT studies performed on patients with coronary artery disease. Indeed, SPECT defects that are documented in viable LV areas may be attributed to real insufficiency of tissue perfusion, while neglecting the influence of cellular metabolic disorders. Reliable information on perfusion is a useful tool when referring patients for specific therapeutic interventions such as coronary revascularization or antianginal medications.
CONCLUSION
Our model provided original information and showed that within myocardial tissue, the kinetics of 201Tl and MIBI across the plasma membrane of myocytes were affected only poorly by low-flow ischemia and not at all by stunning. Our integrative analysis also showed that the slight impairment during ischemia of tracer kinetics through the plasma membrane had no deleterious effects on global myocardial retention of 201Tl and MIBI. Therefore, 201Tl and MIBI are flow-dependent tracers, even in the presence of cellular metabolic disorders related to ischemia or stunning, a property that is useful in clinical practice, particularly for SPECT imaging performed on patients with coronary artery disease.
Acknowledgments
The authors thank Jean-Marc Gravier (Laboratoire de Biophysique, Faculté de Médecine de Nancy), who played a major role in performing the experiments; Henri Boutley (Laboratoire de Biophysique, Faculté de Médecine de Nancy) for technical assistance; Gisèle Gerard (Pall Biomédical, France) for providing leukocyte removal filters; and Isabelle Fries (Laboratoire d’Hématologie Faculté de Pharmacie de Nancy) for the preparation of red blood cell concentrates. This study was supported by grants from ARISC (Association de Recherche et d’Information Scientifique en Cardiologie) and from the Faculty of Medicine of Nancy (Wittner Price).
Footnotes
Received Aug. 3, 2001; revision accepted Dec. 12, 2001.
For correspondence or reprints contact: Adey Ayalew, MSc, Laboratoire de Chirurgie Expérimentale, Faculté de Médecine de Nancy, 54500 Vandoeuvre, France.
E-mail: aayalew{at}yahoo.com