Abstract
The normal myocardium uses primarily fatty acid as its energy source, but, as heart failure develops, the myocardial fatty acid metabolism is limited. In this study, impairment of the lipid metabolism in heart failure was serially evaluated with 123I-(ρ-iodophenyl)-3-(R,S)-methylpentadecanoic acid (BMIPP), a radioiodinated fatty acid analog. Methods: Rapid ventricular pacing was introduced in 10 beagle dogs. Dogs were subjected to hemodynamic assessment and measurement of catecholamine before and after pacing. After 1 wk (group A; n = 4) and 4 wk (group B; n = 6) of pacing, BMIPP was injected directly into the left anterior descending artery; its extraction, retention, and washout rate in the early phase were calculated, and the metabolites in the myocardium were evaluated using high-performance liquid chromatography. These factors were compared with those of healthy control animals (group C; n = 6). Results: The left ventricular ejection fraction and cardiac output decreased significantly in groups A and B after pacing. The pulmonary capillary wedge pressure did not change in group A but increased significantly in group B. Plasma norepinephrine increased progressively as heart failure developed but did not reach statistical significance. The washout rate in the early phase increased, significantly in groups A and B compared with that of group C. Extraction and retention of BMIPP did not change in group A. In group B, extraction tended to decrease and retention decreased significantly compared with that of group C. The levels of full metabolite formed by complete oxidation of BMIPP decreased, and backdiffusion of BMIPP increased significantly in groups A and B compared with that of group C. Myocardial blood flow did not change among the three groups. Conclusion: Our study indicates that myocardial fatty acid oxidation begins to be inhibited and that washout of BMIPP increases in the compensated stage of left ventricular dysfunction but that myocardial extraction and retention of fatty acid are definitely impaired in the advanced stage of heart failure. Therefore, as assessed by BMIPP, the myocardial lipid metabolism is related to the pathophysiology of the development and worsening of heart failure.
Congestive heart failure (CHF) is a common clinical problem confronting physicians and is often the final manifestation of many cardiovascular disorders. Considerable advances in our understanding and management of heart failure have been made recently. However, with increasing life expectancy and decreasing mortality of heart failure, the incidence, prevalence, mortality, and economic costs of the disease are increasing steadily. Therefore, basic research into the fundamental mechanisms accounting for the progression of CHF, with the hope of developing novel therapeutic approaches to alter the progressive course of the disease, has become a priority.
The energy requirements of the normal heart in the basal state are usually met by catabolism of free fatty acid, with minimal contributions by lactate and glucose (1). Previous studies have shown that the heart switches its reliance on glycolysis as the primary pathway for energy production during the development of heart failure (2–4). Little is known about the regulatory mechanisms involved in this cardiac energy substrate switch. Moreover, the role of this metabolic switch as an adaptive versus a maladaptive response and its potential contribution to the transition from compensated left ventricular dysfunction to the overtly failing heart are unknown.
123I-(ρ-iodophenyl)-3-(R,S)-methylpentadecanoic acid (BMIPP) is a radioiodinated fatty acid analog used for myocardial SPECT imaging on the basis of its high cardiac fatty acid metabolism (5). The β-methyl side-branched structure of BMIPP does not affect its conversion to acyl coenzyme A but reduces its susceptibility to β-oxidation. As a result, BMIPP shows superior characteristics for clinical SPECT and has as long a myocardial retention time as those of triglycerides (5,6). We have reported several animal studies using BMIPP and showed that this tracer is useful for evaluating myocardial fatty acid metabolism (7–13).
In this study, we evaluated myocardial fatty acid metabolism using BMIPP in a pacing-induced heart failure dog model that has been used as a well-established, fairly noninvasive model and has proven to produce a stable and chronic state of low-output biventricular heart failure (14–17). We also evaluated how myocardial fatty acid oxidation changed from the early phase of cardiac dysfunction to the final stage of heart failure by changing the pacing protocol.
MATERIALS AND METHODS
Animal Preparations
This study followed the Guidelines for Animal Experiments of Kyoto University established in 1988.
Adult male beagle dogs were studied. Two study protocols were followed. Four dogs were used to study the effects of 1 wk of rapid ventricular pacing (group A); six dogs were used to study the effect of pacing for 4 wk (group B). Six dogs were used as healthy controls (group C). After anesthetization with pentobarbital (25 mg/kg), hemodynamic studies were performed. A thermodilution Swan-Ganz catheter for infants (Baxter Healthcare Co., Deerfield, IL) was inserted under fluoroscopy through the right external jugular vein and positioned in the pulmonary artery. The pulmonary wedge pressure and cardiac output were measured. Pulmonary arteriography was performed for estimation of the left ventricular wall motion and measurement of the left ventricular volume and ejection fraction. At the same time, blood was drawn for measurement of norepinephrine.
After the hemodynamic measurement, a pacemaker lead was introduced through the right external jugular vein and fixed to the apex of the right ventricle. The position of the lead was checked by fluoroscopy. The lead was tunneled to the back and connected to a subcutaneously implanted generator. The pacemaker (Fukuda Denshi Co., Kyoto, Japan) was set at a rate of 220 bpm. After recovery from anesthesia, the dogs were returned to a chronic care facility where they received a standard diet and free access to water.
Experimental Procedure
After overnight fasting, the dogs were anesthetized by intramuscular injection of ketamine hydrochloride (2.5 mg/kg) for the induction; this was followed by intravenous injection of pentobarbital (25 mg/kg) for the maintenance of anesthesia, and blood was withdrawn. Hemodynamic measurement was performed. After endotracheal intubation, the animals were connected to a dual-phase control respirator (Harvard Apparatus, South Natik, MA) that supplied 100% oxygen mixed with room air at 2 L/min. A catheter was inserted into the left femoral artery to monitor blood pressure, and another catheter was inserted into the abdominal aorta through the opposite femoral artery for arterial blood sampling. A triple-lumen intravenous catheter was placed in the femoral vein for fluid infusion and drug administration. A thoracotomy was performed at the fifth intercostal space, and the epicardium was fixed to the thoracic wall in the form of a cradle. The left anterior descending artery was dissected free for radioisotope administration. The great cardiac vein (GCV) was also dissected free and cannulated, and a three-way valve was attached to switch the blood flow to the left appendage of the heart for recirculation or to the open port for venous blood sampling. The colored microspheres were infused through the catheter in the left appendage for measurement of regional myocardial blood flow.
Measurement of Regional Myocardial Blood Flow
The colored microspheres were nonradioactive (E-Z Co. Ltd., Los Angeles, CA), and 7–10 million microspheres were injected into the left appendage with a syringe after sufficient manual mixing with another connected syringe. Blood samples were collected simultaneously from the femoral artery at a rate of 10 mL/min over a period of 90 s. After the dogs were killed, myocardial tissue samples (each piece, 0.5–1.0 g) were collected from 20 sites in the epicardial and endocardial area. Each sample was then treated by routine methods, as reported (18). Microspheres were counted under a microscope at ×100–400 magnification, and the regional myocardial blood flow was quantified.
Measurement of Metabolic Parameters
Blood sampling from the GCV and the abdominal aorta was performed to measure lactate, blood sugar, and nonesterified fatty acids. Lactate consumption was calculated as follows:

Extraction of BMIPP
This study followed our previous protocol (7) and involved collection of all blood from the GCV for 60 s immediately after injection of a mixture of 123I-BMIPP (18.5 kBq) and 125I-bovine serum albumin (18.5 kBq) in 100 μL saline into the left anterior descending artery. The collected blood samples were weighed, and the radioactivity was measured by a well scintillation counter (ARC-350; ALOKA, Tokyo, Japan) with decay correction. The actual radioactive content of 123I and 125I in the samples was calculated using the cross-talk ratio obtained from the 123I standard sample (cross talk from 125I to 123I was negligible). The average flow rate in the GCV was calculated from the weight of the blood samples and the extraction fraction as follows:
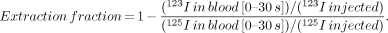
Retention and Metabolism of BMIPP
Immediately after the extraction study, 123I-BMIPP (74 MBq; 0.2 mL) was injected into the left anterior descending artery. All of the blood from the GCV was collected from 0 to 30 s after injection to minimize leakage of untrapped BMIPP into the systemic circulation. Both venous blood from the GCV and arterial blood from the abdominal aorta were collected into heparinized tubes at various time intervals (30 s and 1, 2, 5, 10, 15, and 30 min after injection). Plasma samples were separated by centrifugation at 3000 rpm for 10 min, and the radioactivity of a 0.1-mL aliquot was measured by a well scintillation counter as soon as possible. The remainder of the plasma was extracted twice with a 2:1 mixture of chloroform and methanol (19). The organic layer was then collected and evaporated, and the residue was dissolved in 500 μL methanol for high-performance liquid chromatography (HPLC). An LC-6A chromatographic system (Shimazu Co. Ltd., Kyoto, Japan) with a YMC-Pack ODS column (20 × 150 + 20 × 50 mm; YMC Co. Ltd., Kyoto, Japan) was used for the HPLC analysis. The mobile phase was a mixture of methanol, water, and acetic acid (96:4:1) with a flow rate of 6 mL/min. After injection of the sample, the eluate was collected in 1-min fractions with a fraction collector. The radioactivity of each fraction was then measured with the well scintillation counter with decay correction.
Data Calculation
By fitting time–activity data with a three-exponential curve, the area under the curve (AUC) was calculated using a Newton-Raphson algorithm. The following parameters were also calculated:
Cumulative dose = injected dose × extraction fraction.
Washout dose (0.5–30 min) = AUC of (radioactivity in GCV plasma − radioactivity in arterial plasma) × average flow rate in GCV × ([100 − hematocrit]/100).
Retention fraction at 30 min = 1 − (washout dose/cumulative dose).
Percentage washout in the early phase (8 min) = (washout dose [8 min]/washout dose ([30 min]) × 100.
The percentage cumulative metabolite washout fraction (0.5–30 min) was also calculated.
The plasma metabolite levels were calculated from the total radioactivity in plasma and the fraction of each metabolite obtained by HPLC. Washout of each metabolite from the myocardium was then estimated from the difference in the metabolite levels of arterial and GCV plasma. The extraction of BMIPP from the arterial plasma was taken into consideration as follows:

Metabolite washout was fitted to a three-exponential curve, and the AUC was calculated. The cumulative metabolite washout fraction (1–30 min) was then calculated as follows:

Statistical Analysis
All data are expressed as mean ± SD. Comparisons were performed with the Student t test, paired t test, or multiple comparison test. P < 0.05 was considered statistically significant.
RESULTS
Hemodynamic Data
Hemodynamic data at baseline and after pacing are given in Table 1. After pacing, cardiac output decreased significantly from 3.19 ± 1.08 L/min to 1.71 ± 0.50 L/min in group A (P < 0.05) and from 3.54 ± 1.30 L/min to 1.47 ± 1.06 L/min in group B (P < 0.01). In group A, the pulmonary capillary wedge pressure increased from 6.0 ± 3.6 mm Hg to 8.5 ± 2.6 mm Hg but did not reach statistical significance (P = 0.55). In group B, the pulmonary capillary wedge pressure increased significantly from 7.3 ± 2.4 mm Hg to 19.7 ± 2.7 mm Hg (P < 0.01). After pacing, the pulmonary capillary wedge pressure in group B was significantly higher than that in group A (P < 0.01).
Hemodynamic Parameters at Baseline and After Pacing
Left ventricular end-diastolic volume did not change in group A (33.5 ± 16.2 mL before pacing and 33.8 ± 13.6 mL after pacing) but increased significantly in group B (34.2 ± 8.6 mL before pacing and 47.5 ± 9.3 mL after pacing; P < 0.05). Left ventricular end-systolic volume increased significantly in groups A and B (13.5 ± 6.5 mL to 21.0 ± 8.4 mL and 13.7 ± 5.0 mL to 33.7 ± 6.7 mL, respectively), and it was significantly higher in group B than in group A (P < 0.05). Left ventricular ejection fraction decreased significantly in groups A and B (60.0% ± 3.2% to 37.0% ± 2.2% and 61.0% ± 9.2% to 29.3% ± 1.2%, respectively), and it was significantly lower in group B than in group A (P < 0.01).
Blood Samples
Metabolic parameters and norepinephrine values are summarized in Table 2. Arterial − venous difference (A − V difference) for nonesterified fatty acids did not change between group A (0.05 ± 0.08 mEq/L) and group B (0.12 ± 0.05 mEq/L). Also, the A − V difference for blood sugar did not change between two groups (10.3 ± 9.6 mg/dL in group A and 6.0 ± 4.4 mg/dL in group B). Lactate consumption was also unchanged between both groups (45.3% ± 15.8% in group A and 22.7% ± 13.5% in group B). Plasma norepinephrine increased progressively with time (171 ± 67 pg/mL in group C, 316 ± 42 pg/mL in group A, and 516 ± 477 pg/mL in group B), but these parameters did not achieve statistical significance because of the wide variation of SD.
Metabolic Parameters and Norepinephrine in Groups A and B After Pacing
Myocardial Blood Flow
In group A, the myocardial blood flow was 1.05 ± 0.17 mL/min/g in the whole layer, 1.00 ± 0.20 mL/min/g in the endocardium, and 1.10 ± 0.11 mL/min/g in the epicardium. In group B, the myocardial blood flow was 1.01 ± 0.10 mL/min/g in the whole layer, 0.98 ± 0.11 mL/min/g in the endocardium, and 1.04 ± 0.14 mL/min/g in the epicardium. These differences did not achieve statistical significance.
Metabolism of BMIPP
The extraction, retention, and percentage washout in the early phase are shown in Figure 1. Extraction of BMIPP did not change between group A (77% ± 5%) and group C (74% ± 12%). In group B, extraction of BMIPP (58% ± 20%) decreased but did not reach statistical significance (P = 0.07). In group B, retention of BMIPP (75% ± 13%) decreased significantly compared with that in group C (89% ± 9%; P < 0.05). The percentage washout in the early phase increased significantly in group A (81% ± 5%) and group B (75% ± 15%) compared with that in group C (50% ± 13%; P < 0.01).
Extraction, retention, and percentage washout of BMIPP. Extraction and retention of 1-wk dogs (group A) were almost equivalent to that of control dogs (group C). Extraction of 4-wk pacing dogs (group B) was lower than that of control dogs (group C), but it did not reach statistical significance. Retention of 4-wk pacing dogs (group B) was significantly lower than that of control dogs (group C). Percentage washout of groups A and B was significantly higher than that of group C.
Analysis of metabolites from the myocardium (Fig. 2) revealed that the backdiffusion of BMIPP of group A (75% ± 13%) and group B (61% ± 12%) was significantly higher than that of group C (25% ± 8%; P < 0.01). α-Oxidation metabolites of groups A and B (5% ± 3% and 7% ± 4%, respectively) were significantly lower than those of group C (27% ± 4%; P < 0.01). β-Oxidation metabolites of groups A and B (20% ± 10% and 31% ± 10%, respectively) were also significantly lower than those of group C (48% ± 7%; P < 0.01).
Percentage washout values of radioactive metabolites from myocardium. In groups A and B, levels of α-oxidation metabolite (14-(ρ-iodophenyl)-2-(α)-R,S-methyltetradecanoic acid) and full-oxidation (2-(ρ-iodophenyl)acetic acid) and intermediate metabolites were significantly lower than that of group C. Level of backdiffusion of nonmetabolized BMIPP of groups A and B was significantly higher than that of group C.
DISCUSSION
In this study, 1-wk rapid ventricular pacing produced left ventricular dysfunction, and 4-wk pacing produced severe CHF corresponding to subset IV of Forrester et al. (20) in humans. Analysis of the BMIPP metabolism revealed that the extraction tended to decrease in group B and also showed a significantly lower retention in that group, with increased washout in the early phase and backdiffusion in groups A and B.
BMIPP is a radioiodinated fatty acid analog and it has a methyl group in the β-3 position. The β-methyl side-branched structure does not affect conversion of BMIPP to acyl coenzyme A but reduces its susceptibility to β-oxidation. Therefore, BMIPP is long trapped in the myocardium, permitting metabolic imaging with SPECT (5,6). We have reported several animal studies using the same method as that used in this study (7–13). In a study using etomoxir (8), which is one of the carnitine palmitoyltransferase I inhibitors and inhibits the transport of long-chain lipids into the mitochondria, we showed that early washout and backdiffusion of BMIPP increased and β-oxidation of BMIPP was inhibited, suggesting that BMIPP was suitable for assessing mitochondrial dysfunction. This study also showed that early washout and backdiffusion of BMIPP increased and that β-oxidation of BMIPP was inhibited at 1 and 4 wk after pacing, suggesting that mitochondrial dysfunction in the myocardium occurred in the early phase of left ventricular dysfunction and continued until the advanced stage of heart failure. We have also shown that the uptake of BMIPP correlated well with the lipid metabolism and tissue ATP levels using the ischemic model, proving useful for differentiating infarcted myocardium from viable ischemic myocardium (9). In this study, we found that the myocardial uptake of BMIPP was severely inhibited, suggesting decreased myocardial energy production by fatty acids.
Pacing-induced heart failure was first described by Whipple et al. (21) in 1962. Although these investigators initially devised this model to mimic tachycardia-induced cardiomyopathy in humans, it has been used increasingly as a well-established, fairly noninvasive model that has proven to produce a stable and chronic state of low-output, biventricular, edematous heart failure (14–17). By varying the pacing protocol, different severities and stages of heart failure can be induced. In this study, ventricular pacing at a rate of 220 bpm continued for 1 and 4 wk. The 1-wk pacing model was thought to correspond to a model of early asymptomatic left ventricular dysfunction described by Redfield et al. (22). Four-week pacing produced overt CHF, hemodynamically corresponding to subset IV of Forrester et al. (20) in humans. This study showed that β-oxidation of BMIPP was inhibited and that backdiffusion of BMIPP increased in the stage of early left ventricular dysfunction. This finding suggests that fatty acid metabolism in the mitochondria was inhibited in the early phase of cardiac dysfunction, but the extraction and retention of BMIPP did not change significantly, suggesting that incorporation in the endogenous lipid pool was not impaired in this phase. In contrast, extraction of BMIPP tended to decrease and retention of BMIPP decreased significantly in the stage of overt heart failure. These findings suggested that the energy store as triglyceride and the uptake of fatty acid into myocardium decreased. Hence, the myocardium was in an energy-deficient condition in the final stage of heart failure.
The precise mechanisms responsible for the contractile dysfunction and structural changes of pacing-induced heart failure are not known. Several mechanisms have been proposed (23): myocardial energy depletion and impaired energy use, myocardial ischemia, abnormalities of cardiac calcium regulation, and myocyte and extracellular matrix remodeling. Wilson et al. (16) reported that acute and chronic pacing were associated with persistently elevated coronary blood flow with no significant change in myocardial lactate and O2 extraction—that is, no evidence of ischemia. In this study, we also found that myocardial blood flow and myocardial lactate consumption did not change between group A and group B. This showed that myocardial ischemia was not found in this model. Results of this study suggest that the myocardial fatty acid metabolism in the mitochondria might be inhibited in the early phase of cardiac dysfunction and that the uptake and energy store might decrease in the overt heart failure stage. Therefore, the myocardial fatty acid metabolism might be closely related to cardiac dysfunction. Sack et al. (24) found that, although medium-chain acyl coenzyme A dehydrogenase (the key enzyme in the fatty acid β-oxidation) messenger RNA levels were downregulated in hypertrophy and heart failure stages in SHHF/Mcc-facp rats, the enzyme activities and protein levels did not decrease significantly until heart failure ensued. These results indicate that a gene-regulatory pathway is involved in the control of cardiac energy production during the development of heart failure. Such a pathway might reflect the metabolic change of BMIPP in this study. How impairment of myocardial fatty acid metabolism induces cardiac dysfunction is unclear. However, Kelly and Strauss have documented that defects in the transport, mitochondrial uptake, and β-oxidation of long-chain fatty acids cause cardiomyopathy in infants and children (25); thus, impairment of myocardial fatty acid metabolism might be one of the mechanisms responsible for heart failure.
Sudden cardiac death accounts for ∼30%–50% of total mortality in patients with heart failure. Although ventricular arrhythmias are responsible for most of these deaths, the underlying mechanisms remain poorly understood (26). Repolarization abnormalities, enhanced dispersion of repolarization, and reduced outward K+ currents are some of the proposed mechanisms. Pak et al. (27) showed that pacing-induced heart failure exhibited malignant arrhythmia and sudden cardiac death. This finding suggests that pacing-induced heart failure is suitable for studying the mechanisms and therapy of sudden cardiac death in heart failure and that impairment of the myocardial fatty acid metabolism might be related to the mechanism of sudden cardiac death in heart failure. In fact, Bonnet et al. (28) reported a series of 24 children in whom arrhythmias or conduction defects were the predominant presenting symptom of various fatty acid oxidation disorders.
CONCLUSION
In the early ventricular dysfunction stage, washout in the early phase and backdiffusion of BMIPP increased and β-oxidation of BMIPP decreased. In addition, in the overt heart failure stage, extraction of BMIPP tended to decrease and retention of BMIPP decreased. These findings suggest that myocardial lipid metabolism is related to the pathophysiology of the development and worsening of heart failure. The metabolism of BMIPP might be useful in evaluating the pathophysiology, extent, and prognosis of heart failure.
Acknowledgments
The authors thank Daniel Mrozek for reading the manuscript and Nihon Medi-Physics Co. Ltd. (Tokyo, Japan) for product support and encouragement.
Footnotes
Received Mar. 20, 2000; revision accepted Jun. 30, 2000.
For correspondence or reprints contact: Ryuji Nohara, MD, Department of Cardiovascular Medicine, Graduate School of Medicine, Kyoto University, 54 Kawara-cho, Shogoin, Sakyo-ku, Kyoto 606-8397, Japan.








