Abstract
The Code of Federal Regulations, title 10, part 35.75 (10CFR35.75), provides greater latitude and flexibility in the dosing and management of outpatients treated with therapeutic 131I than did preceding regulations. Prescribing physicians should consider applying these new regulations to enhance patient convenience and lower the cost of managing appropriate outpatients. Managed care organizations and third-party payers may require that all eligible patients be treated as outpatients or that justification for hospital admission be specifically documented. To facilitate application of the code and guidelines, maximum 131I doses for patients undergoing thyroid remnant ablation, therapy for metastatic or recurrent thyroid cancer, or therapy for hyperthyroidism have been calculated and summarized in tables. Methods: A model was developed that calculates the maximum dose of 131I that may be dispensed to an outpatient. This model complies with 10CFR35.75. The maximum dose is calculated as a function of 5 variables: the occupancy factors for 3 periods after dose administration, the fractional uptake of 131I by residual thyroid tissue or metastasis, and the duration of constrained activity. Occupancy factor, a key new concept in the regulatory guidelines, is a physician estimate of the time that a treated patient will be near the individual with whom the patient will spend the most time after treatment. The model also considers 3 constants: the effective half-life of 131I during the preequilibrium period, and the effective half-lives of 131I in both the thyroidal component and the extrathyroidal component during the equilibrium period. Tables for maximum allowable patient 131I doses were derived on the basis of this model. Results: Through dosing charts, maximum 131I therapy doses may easily be calculated. Most outpatients undergoing thyroid remnant ablation, therapy for metastatic or recurrent thyroid cancer, or therapy for hyperthyroidism may be treated with 7400 MBq (200 mCi) 131I or more. Conclusion: If the prescribing physician understands the concept of occupancy factor and how to use the dosing charts, our model facilitates application of and adherence to 10CFR35.75.
The new Code of Federal Regulations, title 10, part 35.75 (10CFR35.75), provides greater latitude and flexibility in the management of outpatients treated with 131I than did preceding regulations (1). Prescribing physicians should consider applying these new regulations to enhance patient convenience and lower the cost of managing appropriate outpatients. Managed care organizations and third-party payers may require that all eligible patients be treated as outpatients or that justification for hospital admission be specifically documented.
The new regulations were promulgated to reflect a greater sensitivity to realistic situations and provide greater physician flexibility in patient treatment while protecting the public from exposure to unsafe levels of radiation. The unique living situation of each patient, as well as his or her ability to adopt a small set of social limitations for several days, are the basis for determining the maximum prescribable 131I dose. Tables were derived to help physicians determine the maximum dose, Qmax, that a patient may receive for safe release to a structured outpatient environment.
Until recently, patients receiving more than 1100 MBq (30 mCi) 131I required hospitalization. The new regulation allows patients to be released as outpatients on the basis of an estimate of the radiation dose to the person who will bear the greatest exposure. The calculations are based on the percentage of 131I uptake, the effective half-lives of 131I, and the occupancy factor (OF). OF is a physician estimate of the patient's proximity to people at home or in the community during and after a period of constrained social interaction. The physician estimates the time that the patient will be near the person with whom the patient will spend the most time after dose administration. Qmax calculations can be individualized by allowing the prescribing physician to assign a separate OF for each of 3 intervals after the administration of the dose. These 3 intervals are the preequilibrium period (first 8 h after dose administration) the constrained-activity period (intentional constraint of patient's social interaction after the preequilibrium period), and the unconstrained-activity period (the following infinite period). The prescribing physician assigns these OFs on the basis of the patient's lifestyle.
Our model assumes that after ingestion of 131I, an 8-h preequilibrium period exists during which little biologic elimination of 131I occurs. The effective half-life of 131I during this period is considered constant, although some interpatient variability exists and is estimated to be 0.8 times the physical half-life, or 6.43 d (2). The estimate of the regulatory guide—that the effective half-life of 131I during the preequilibrium period is 0.8 times the physical half-life of 131I (0.8 × 8.04 = 6.432 d)—is not substantiated. The term “half-life” refers to an equilibrium status that obeys first-order kinetics; thus, the term is not applicable to the preequilibrium period of the model. However, most conservatively, if the effective half-life of 131I during the preequilibrium period were equal to its physical half-life, the calculated Qmax, using Equation 1, would be reduced by less than 0.5%. Because this amount makes no practical difference in dose calculations, the estimate of the regulatory guide is used in this model for consistency.
After the preequilibrium period, the remaining 131I is considered to be divided between the thyroidal component and the extrathyroidal component. The effective half-life of 131I in each of these components is different. The effective half-lives of 131I for thyroidal and extrathyroidal components also vary from patient to patient but were assumed to be constant in this model and were assigned values of 7.3 and 0.32 d, respectively (2,3). The model was developed using an effective 131I half-life of 7.3 d in the thyroidal component. The regulatory guide uses this value for post-thyroidectomy patients but 5.2 d for hyperthyroid patients. The model uses 7.3 d for both post-thyroidectomy patients and hyperthyroid patients because appropriate doses for hyperthyroid patients are within the Qmax values calculated using the 7.3-d effective half-life, which is more conservative. The 0.32-d value is from the regulatory guide. We acknowledge that this value varies from patient to patient and may be prolonged in hypothyroid patients because of decreased gastrointestinal motility and reduced glomerular filtration rate. An effective half-life of 0.32 d is used for consistency.
In addition to the 3 constants, the model comprises the following 5 variables: the fractional thyroid 131I uptake, the duration of the period of constrained social activity, and the OFs for the 3 periods (the preequilibrium, OFp; the constrained, OFc; and the unconstrained, OFuc). A function of the 3 constants and 5 variables is derived that calculates the maximum prescribable dose that, in compliance with 10CFR35.75, will limit exposure of any other individual to 5 mSv or less per patient release (4).
Application of this model allows a physician to easily implement treatment plans for outpatients using 131I therapy doses greater than 1100 MBq (30 mCi). The tables and equations provide the legally required documentation and criteria for discharge of the patient. The use of this model in clinical practice does not preclude alternative release criteria but is predicated on the new Nuclear Regulatory Commission regulations, which are applicable in Nuclear Regulatory Commission states only (2). The use of this model or alternative release criteria should be reviewed with the state regulatory agency in agreement states.
If the fractional uptake of iodine is known or estimated, if the OF values are assigned, and if a period of constrained patient activity is defined, Qmax can be determined from Table 1. Qmax may also be calculated from an equation and tables (Eq. 4; Tables 2 and 3) or derived from a single equation (Eq. 5). These equations and tables are based, in part, on approximations and assumptions used in the regulatory guide and fully comply with 10CFR35.75. Although conceptually similar to the regulatory guide, this model is more comprehensive.
Qmax
Exposure per Millicurie During Constrained Period
Exposure per Millicurie During Unconstrained Period
Internal contamination of nonpatients is relevant in considering whether a patient may be sent home. Although studies show that those exposed to patients receiving 131I therapy incur relatively little internal radiation contamination and that it may be overlooked when calculating the exposure of the unintended individual (2,4), patients should be told how internal contamination of others may occur. This is most important during the first 2–3 d after treatment, when a relatively high biologic clearance from the extrathyroidal component occurs. Patients should be told what constitutes internal contamination and how to prevent it during the first 2 or 3 d after treatment. These instructions should be specific to each patient but should always include points such as having sole use of a bathroom, flushing the toilet twice, and using disposable eating utensils.
MATERIALS AND METHODS
To derive the equation that is the basis for the model, exposure to an individual standing near a constant source of radiation is calculated:
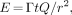 Eq. 1where E = unintended exposure (millisieverts) to individuals other than the patient, Γ = exposure rate constant for 131I = (2.2 R × (cm)2)/(mCi × h) (5) = (0.0528 mSv × m2)/(mCi × d) (assuming 1 R = 10 mSv (2)), t = duration of exposure (days), Q = activity (millicuries), and r = distance from source (meters).
Eq. 1where E = unintended exposure (millisieverts) to individuals other than the patient, Γ = exposure rate constant for 131I = (2.2 R × (cm)2)/(mCi × h) (5) = (0.0528 mSv × m2)/(mCi × d) (assuming 1 R = 10 mSv (2)), t = duration of exposure (days), Q = activity (millicuries), and r = distance from source (meters).
In clinical practice, the calculation of exposure to individuals in daily contact with a patient who has received 131I therapy becomes more complex (6). The reason is 4-fold: the biodistribution of 131I varies among patients, the activity within the patient decreases by physical decay and biologic elimination, the patient's social activity is intentionally constrained for a predetermined period, and the distance between an individual and the patient varies over time.
These concerns are addressed in the model on the basis of assumed thyroid iodine uptake, effective half-lives, period of constrained activity after the preequilibrium period, and OF values. Each of these concerns is addressed separately in the following sections.
Thyroid 131I Uptake
The fractional uptake of 131I by thyroid or thyroidlike tissue in the neck is easily measured. When iodine-avid thyroid metastatic lesions occur outside the thyroid bed, the measurement of fractional iodine uptake becomes difficult and, at times, may be only roughly estimated. Most patients with a history of thyroid carcinoma present seeking either ablation of only a small, postoperative remnant of normal thyroid tissue or treatment of a small mass of locally recurrent disease. These patients may be treated empirically as if their uptake were 0.05 (5%). Patients who present with larger amounts of residual thyroid tissue or a relatively large mass of iodine-avid recurrent thyroid carcinoma will require determination of fractional iodine uptake before treatment. Fractional uptake measurements are also required when treating hyperthyroidism with a dosage greater than 1100 MBq (30 mCi) 131I.
Effective Half-Lives
The term “half-life” implies first-order kinetics; the rate of decay is proportional to the amount of substance remaining. The effective half-life of 131I assumes that the rate of 131I loss is proportional to the amount of 131I remaining or is equal to a first-order rate constant times the amount of 131I remaining. If the rate constant representing the biologic loss of 131I and the rate constant representing 131I physical decay are added, the sum equals the effective rate constant (keff), which represents total loss from both biologic elimination (kbio) and physical decay (kphy): keff = kbio + kphy. Because the first-order rate constants are proportional to the reciprocals of the half-lives, 1/Teff = 1/Tbio + 1/Tphy, where Teff = the effective half-life, Tbio = the biologic half-life, and Tphy = the physical half-life. The effective half-life represents the half-life of 131I within the body and is a function of both biologic elimination and physical decay.
Three different effective half-lives are used in the model: the preequilibrium (Tp), thyroidal (Tth), and extrathyroidal (Te) component half-lives. The model assumes that after ingestion of 131I, a preequilibrium period of one-third day (8 h) exists during which little biologic loss of 131I occurs (2). The effective half-life during this period is estimated to be 0.8 times the physical half-life of 131I, or (0.8)(8.04) = 6.432 d (2). In Equation 1, when Q decreases as a function of time, the product Q × t is replaced by the integral ∫0 tQt dt. In the preequilibrium period for this example, this integral is Q0 ×  × e−[(ln2)/(0.8)(8.04)]t dt and equals 0.3274 Q0 (mCi-day). Q0 (mCi) is the initial patient dose. Substituting 0.3274 Q0 (mCi-day) into Equation 1 and normalizing to unit distance (r = 1 m), Ep (mSv/mCi) = Γ(0.327)Q0/r2 = (0.0528)(0.327)Q0/12 = 0.0173 mSv/mCi, where Ep = preequilibrium exposure (mSv/mCi), Γ = 0.0528 (mSv × m2)/(mCi × d), and Q0 = initial dose.
× e−[(ln2)/(0.8)(8.04)]t dt and equals 0.3274 Q0 (mCi-day). Q0 (mCi) is the initial patient dose. Substituting 0.3274 Q0 (mCi-day) into Equation 1 and normalizing to unit distance (r = 1 m), Ep (mSv/mCi) = Γ(0.327)Q0/r2 = (0.0528)(0.327)Q0/12 = 0.0173 mSv/mCi, where Ep = preequilibrium exposure (mSv/mCi), Γ = 0.0528 (mSv × m2)/(mCi × d), and Q0 = initial dose.
Preequilibrium exposure calculates the dose an individual would receive if that person were 1 m from the patient for the entire 8 h after initial dosing, or 0.0173 Q0 (mSv). The activity remaining within the patient at 8 h would be Q0e−[ln2/(3)(0.8)(8.04)] = 0.965 Q0.
After this preequilibrium 8-h component, the model is represented by a bicomponent system: the thyroidal component and the extrathyroidal component. The effective half-life of 131I in the thyroidal component is estimated to be 7.3 d, and that in the extrathyroidal component is estimated to be 0.32 d (2,3). When the bicomponent model begins, the activity remaining is 0.965 Q0. Each component is considered separately.
The exposure an individual would receive from the thyroidal component by remaining 1 m from the patient for an infinite time is Eth(mSv) = 0.965ΓUQ0/r2 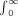 e−[(ln2)t/Tth] dt = 0.537UQ0, where Eth = exposure from the thyroidal component = 0.537UQ0 (mSv), U = the fractional thyroid uptake of 131I, Tth = 7.3 d, and r = 1 m. Similarly, the exposure from the extrathyroidal component, in which Te = 0.32 d, is Ee (mSv) = 0.0236(1 − U)Q0.
e−[(ln2)t/Tth] dt = 0.537UQ0, where Eth = exposure from the thyroidal component = 0.537UQ0 (mSv), U = the fractional thyroid uptake of 131I, Tth = 7.3 d, and r = 1 m. Similarly, the exposure from the extrathyroidal component, in which Te = 0.32 d, is Ee (mSv) = 0.0236(1 − U)Q0.
Period of Constrained Social Activity
This model considers the patient's social interactions to be intentionally constrained for a time after the preequilibrium period. This period of constrained activity is usually only 2 d for patients undergoing thyroid remnant ablation or therapy for local recurrence of thyroid cancer. Little iodine-avid tissue exists in these patients, and their extrathyroidal iodine clears relatively quickly. However, longer periods of constrained activity may be more appropriate in certain cases when the fractional uptake of 131I is higher.
Exposure from the thyroidal and extrathyroidal components is divided into the period of constrained activity and the following infinite period of unconstrained activity. One minus the fraction of the dose taken up by thyroid tissue is the remainder of the dose, or the fraction not taken up by thyroid tissue. If the period of constrained activity (C), in days, is considered, Eth(c) = 0.537UQ0(1 − e−[(ln2)C/Tth]) = 0.537UQ0(1 − e−0.095C) = exposure from the thyroidal component during the period of constrained activity, and Ee(c) = 0.0236(1 − U)Q0(1 − e−[(ln2)C/Te]) = 0.0236(1 − U)Q0(1 − e−2.08C) = exposure from the extrathyroidal component during the period of constrained activity.
The exposure to a specific individual after the period of constrained activity until complete decay is calculated as follows: Eth(uc) = 0.537UQ0e−0.095C = exposure from thyroidal component after the period of constrained activity until complete decay, and Ee(uc) = 0.0236(1 − U)Q0e−2.08C = exposure from extrathyroidal component after the period of constrained activity until complete decay. The fractional thyroid uptake of 131I can be measured before treatment; it is assumed to be 0.05 for thyroid remnant ablation, provided that thyroidectomy is nearly complete (1).
Total Exposure
Because the effective half-lives of 131I in the 2 components, thyroidal and extrathyroidal, are considerably different, the potential exposure to specific individuals is very much a function of the dose biodistribution within the patient, or, more specifically, the fractional thyroid uptake. The total exposure (millisieverts) received by a person who spends 100% of his or her time with the patient comprises 5 components: the preequilibrium component, the thyroidal and extrathyroidal components during the periods of constraint and of unconstraint, and the thyroidal and extrathyroidal components after the period of constraint, or E = Ep + Eth(c) + Ee(c) + Eth(uc) + Ee(uc). When terms are combined, Ep = 0.0173 mSv, Ec = (Eth(c) + Ee(c)) = exposure during the constrained period,Euc = (Eth(uc) + Ee(uc)) = exposure during the unconstrained period, and:
 Eq. 2
Eq. 2
OF Values
OF is a conservative physician-estimate of the fraction of time that an individual is near the patient. OF, as used in the regulatory guide, is part of a model that assumes a person is either 1 m from the patient or far from the patient. The radiation exposure a person receives from 131I activity in the patient varies as the inverse of the square of the person's distance from the patient. For facilitation of exposure estimates, the distance from the patient is considered to be 1 m or infinite, and OF should conservatively estimate the fraction of time that the distance is “1 m.” During the period of constrained activity, an OFc of 0.25 is considered conservative. In other words, the person who is most with the patient is estimated to be no more than 1 m from the patient no more than 25% of the time and to not be in the presence of the patient for the remaining time. If OF is used as a criterion for patient discharge, the patient must follow specific instructions. For example, an OF of 0.25 may be assigned for the constrained period if the patient maintains a prudent distance from others, sleeps alone in a room, does not travel by airplane or mass transportation, does not travel on a prolonged automobile trip with others, and drinks plenty of fluids. An OF of 0.125 may be used if the patient lives alone and has few visitors. In either case, the patient should receive detailed, personalized instructions about 131I excretion and contamination pertaining to the living situation and should also receive the name and telephone number of a person knowledgeable in radiation safety.
Section 8.39 of the regulatory guide suggests that an OFp of 0.75 be assigned to all patients during the preequilibrium period. This takes into account the patient's transportation home after dosing. The patient may be near other individuals during the trip, which may last several hours. The regulatory guide assumes that a patient spends 6 of the first 8 h after dosing 1 m from 1 or more individuals. We consider this assumption to be very conservative, but it has merit under special circumstances.
If the prescribing physician believes that a lower OF may be appropriate for the preequilibrium period, assigning the same OF to the preequilibrium period as is assigned to the constrained equilibrium period may be reasonable. For example, a patient may live only a short distance from the treatment facility and may drive home alone. If the patient requires a larger dose than the living conditions would allow if he or she were to return home immediately, the patient may be hospitalized for the preequilibrium period. This short hospitalization makes possible a larger allowable dose, because OFp may be assigned a value of zero provided that the person with whom the patient will spend the most time after discharge is not in contact with the patient during the hospitalization. If the patient is alone, will return home alone, and will remain alone for the first 8 h, an OF of zero (OFp = 0) may be appropriate as well.
For the period of constrained activity, most patients can be assigned an OFc of 0.25. An OFuc of 0.5 is usually appropriate for individuals who sleep together. The assignment of OFp, OFc, and OFuc must be based on the living conditions of the specific patient.
The application of appropriate OFs to the components of Equation 2 gives rise to:
 Eq. 3
Eq. 3
If Emax is set at 5 mSv, which is the new regulatory maximum for unintended exposure to any individual, then 5 mSv = Qmax (mCi) [OFp(0.0173) + OFcEc + OFucEuc] (mSv/mCi),
 Eq. 4
Eq. 4
 Eq. 5
Eq. 5

In this model, the maximum allowable 131I dose that may be administered to an outpatient is a function of 5 variables. Three of these variables are OFp, OFc, and OFuc, which should be assigned on the basis of the patient's lifestyle (constrained and unconstrained). The fourth variable, the fractional uptake of 131I in thyroid tissue or metastatic lesions, should be measured or estimated. A fractional uptake of 0.05 may be assigned to post-thyroidectomy patients provided that the thyroidectomy was nearly complete. The fifth variable is the time after the preequilibrium period that the patient's lifestyle will be constrained, that is, the constrained portion of the equilibrium period. Once these parameters have been established, the maximum allowable dose may be found in Table 1, calculated using Equation 4 and Tables 2 and 3, or calculated directly using Equation 5: Qmax = 5/[OFp(0.0173) + OFc(0.537U(1 − e−0.095C) + 0.0236(1 − U)(1 − e−2.08C)) + OFuc(0.537Ue−0.095C + 0.0236(1 − U)e−2.08C)].
RESULTS
Thyroid Remnant Ablation
131I Qmax is easily calculated on the basis of the patient's assigned OFp, OFc, and OFuc, the duration of constrained activity, and fractional thyroid uptake of 131I (Table 1, Tables 2 and 3 and Eq. 4, or Eq. 5). Patients who have undergone an uneventful thyroidectomy may be assigned an uptake of 0.05 as a conservative estimate (2). If more than the usual amount of residual thyroid tissue may remain in the neck (e.g., if surgery was difficult or if the thyroid-stimulating hormone is < 20 μIU/mL), iodine uptake should be measured and the patient's Qmax should be based on this uptake value. In our model, the patient's iodine burden is plotted as a function of time (Fig. 1). The 131I burden is rapidly cleared in thyroid ablation patients with low thyroid uptake. Relative activity is also plotted as a function of both uptake and time (Fig. 2). The character of the time–activity curve changes with changes in uptake.
131I ablation. Relative activity remaining in patient is plotted as function of time.
Relative activity plotted as functions of both time and percentage uptake.
In case 1, a 45-y-old man who underwent an uneventful thyroidectomy for papillary thyroid carcinoma presents for ablation of remnant thyroid tissue. He lives approximately 400 km (250 mi) from the treatment facility and will be driving home with his wife. He lives alone with her. They will sleep in separate rooms for the following 2 nights. The period of constraint will be 2 d, beginning 8 h after dosing. Their living conditions will be such that OF values may be assigned as follows: OFp = 0.75, OFc = 0.125, and OFuc = 0.5. What is Qmax for these conditions?
A fractional uptake of 0.05 is assumed in post-thyroidectomy patients. Using Table 1, Qmax = 6700 MBq (181 mCi). Using Tables 2 and 3 and Equation 4, Ec = 0.027, Euc = 0.023, and Qmax = 5/(OFp(0.0173) + OFcEc + OFucEuc) = 5/((0.75)(0.0173) + (0.125)(0.027) + (0.5)(0.023)) = 6700 MBq (180 mCi).
In case 2, a 29-y-old woman presents for ablation of remnant thyroid tissue. She is single and lives with her 6-y-old daughter. Her daughter attends school 8 h per day. The patient will be dosed early Monday morning. She lives near the hospital and will provide her own transportation home, where she will remain alone for the following 8 h. She and her daughter sleep in separate bedrooms. Her living conditions will be such that the following OF values are appropriate: OFp = 0.0, OFc = 0.25, and OFuc = 0.25. A period of constraint of 2 d is assigned. Because OFc = OFuc, assignment of a period of constraint is mathematically unnecessary; however, a period of constraint stresses to the patient that his or her behavior during the first 2 d is important in limiting exposure to others. What is Qmax for these conditions?
Fractional uptake is 0.05. Using Table 1, Qmax = 15,000 MBq (406 mCi), and 406/5 = 3000 MBq (81 mCi). We recommend dividing Qmax by 5 if OF values pertain to a child 8 y old or less or to a pregnant woman. Using Tables 2 and 3 and Equation 4, Ec = 0.027, Euc = 0.023, and Qmax = 5/(OFp(0.0173) + OFcEc + OFucEuc) = 5/(0 + 0.25(0.027) + 0.25(0.023)) = 14,800 MBq (400 mCi), and 400/5 = 3000 MBq (80 mCi).
Therapy for Hyperthyroidism
Thyroid iodine uptake should be measured on all hyperthyroid patients before 131I therapy, especially if the required dose may exceed 1100 MBq (30 mCi). Hyperthyroid patients rarely require doses exceeding 1100 MBq (30 mCi). However, if a patient does require more than 1100 MBq (30 mCi) 131I, Qmax may be found using the tables and equations. Residual 131I activity is plotted as a function of time if thyroid iodine uptake is 0.35 (Fig. 3).
131I hyperthyroidism therapy. Relative activity remaining in patient is plotted as function of time.
In case 3, a 73-y-old woman was treated with 1100 MBq (30 mCi) 131I for toxic multinodular goiter 1 y previously. She remains clinically and chemically hyperthyroidal, although 131I uptake is only 0.20. She lives with only her husband, and they usually sleep in the same bed. The patient agrees to a 2-day period of constraint during which she will sleep alone. The patient's living conditions justify use of the following OF values: OFp = 0.25, OFc = 0.25, and OFuc = 0.5. What is Qmax for these conditions?
Table 1 is not used, because fractional uptake is 0.20. Using Tables 2 and 3 and Equation 4, Ec = 0.037, Euc = 0.089, and Qmax = 5/(OFp(0.0173) + OFcEc + OFucEuc) = 5/((0.25)(0.0173) + (0.25)(0.037) + (0.5)(0.089)) = 3200 MBq (86 mCi).
In case 4, a 25-y-old woman with Graves' disease presents for 131I treatment. Her 131I thyroid uptake is 0.92, and the gland is estimated to weigh 50 g. She is single and lives alone. She resides only a short distance from the hospital and will be driven home by her mother. She can fulfill requirements for the following OF values: OFp = 0.25, OFc = 0.125, OFuc = 0.25. She will maintain a constrained lifestyle for 2 d. What is Qmax for these conditions?
Table 1 is not used, because fractional uptake is 0.92. Using Tables 2 and 3 and Equation 4, and rounding fractional uptake up to 0.95, Ec = 0.089, Euc = 0.422, and Qmax = 5/(OFp(0.0173) + OFcEc + OFucEuc) = 5/((0.25)(0.0173) + (0.125)(0.089) + (0.25)(0.422)) = 1500 MBq (41 mCi). This dose far exceeds the most appropriate dose this patient should receive. This example is included to emphasize that these equations and tables set upper limits allowable in dosing outpatients with 131I; the equation and tables do not necessarily determine the most appropriate dose.
Treatment of Recurrence
Treatment of a recurrence of thyroid carcinoma is similar to thyroid bed ablation, in that the fractional uptake by metastases is usually less than 0.05. In the rare case of a fractional uptake that is expected to possibly exceed 0.05, the estimation should be based on the appearance of the total-body 131I scan.
In case 5, a 36-y-old woman who was previously treated with 4600 MBq (125 mCi) 131I now presents with metastases to lung and bone. 131I uptake by the metastases is assumed to be less than 0.05 after examination of the 131I total-body scan. The patient lives at home with her husband. She lives only a short distance from the hospital at which she will receive 131I treatment, and she will drive herself home immediately after treatment. She is well known to the prescribing physician, who believes she is reliable. She agrees to follow parameters appropriate for assignment of an OFc of 0.125 for 2 d of constrained activity. Her living conditions will be such that the following OF values are appropriate: OFp = 0.25 and OFuc = 0.5. What is Qmax for these conditions?
Using Table 1, Qmax = 9800 MBq (264 mCi). Using Tables 2 and 3 and Equation 4, Ec = 0.027, Euc = 0.023, and Qmax = 5/(OFp(0.0173) + OFcEc + OFucEuc) = 5/((0.25)(0.0173) + (0.125)(0.027) + (0.5)(0.023)) = 9600 MBq (260 mCi).
In case 6, a 33-y-old woman with a history of papillary thyroid carcinoma treated by thyroidectomy and 5600 MBq (150 mCi) 131I ablation of remnant thyroid tissue recently underwent a total-body scan using 74 MBq (2 mCi) 131I. A small area of increased uptake in the neck was noted, which was consistent with recurrence. She returns in 2 wk for 131I therapy. She lives with her husband and 4-y-old son. She will sleep alone for the first 2 nights and will follow other constraints such that the following OF values are appropriate for her husband: OFp = 0.25, OFc = 0.25, and OFuc = 0.5. Her son will live at his grandparent's home and have no contact with his mother for a time after treatment. What Qmax for her will limit her husband's exposure to 5 mSv?
Fractional uptake is 0.05. Using Table 1, Qmax = 8300 MBq (224 mCi). Using Tables 2 and 3 and Equation 4, Ec = 0.027, Euc = 0.023, and Qmax = 5/(OFp(0.0173) + OFcEc + OFucEuc) = 5/((0.25)(0.0173) + (0.25)(0.027) + (0.5)(0.023)) = 8200 MBq (221 mCi).
DISCUSSION
The equations and tables provide a means of justifying the release of patients treated with relatively high doses of 131I while conforming to the new 10CFR35.75. This will facilitate greater patient convenience and reduce hospital costs while maintaining a high level of radiation safety to members of the community. The equations and tables are appropriate for almost all patients requiring high-dose 131I therapy.
Patients with a large metastatic load with a high affinity for iodine present somewhat of a problem because the required dose is high and the thyroidal component is large. In such a patient, a relatively large iodine burden will be retained for a relatively long period. The actual fractional uptake of iodine may also be difficult to estimate when metastases are widespread. Unfortunately, the period of constrained activity will be quite long in these patients if treated as outpatients. Similarly, their hospital stay could be fairly prolonged while they await unconstrained-release criteria. Fortunately, these patients are encountered infrequently. We believe they should be managed on a case-by-case basis.
Limitations
The entries in the tables should not be interpolated, because they do not follow a linear relationship. If an uptake value is between those provided in the tables, the more conservative value should be used.
Doses calculated using the equations and tables are the maximums that outpatients may receive. The most appropriate dose for a given patient should be determined by the prescribing physician. The tables and equations are for determining whether the appropriate dose may be used in an outpatient setting.
The equations and tables were derived from assumptions in the regulatory guide (2). Qmax values may be calculated by other methods, and other methods may be especially required in unusual circumstances such as renal failure or other conditions that may increase the effective half-life of the extrathyroidal component.
Conditions for Immediate Patient Release
Maximum permissible doses for various levels of 131I thyroid uptake and OFs are provided in Table 1 and may be calculated using Equation 4 and Tables 2 and 3 or using Equation 5. Most patients receiving more than 1100 MBq (30 mCi) 131I may be released immediately if the following conditions are met. First, the unintentional exposure of any individual other than the patient may not exceed 5 mSv per release (1 mSv if the individual is pregnant or is 8 y old or less). Second, the patient must receive both verbal and written instructions, and the instructions must be explained to the patient. The physician should be reasonably certain that these instructions can and will be followed (patients receiving more than 260 MBq [7 mCi] but less than 1100 MBq [30 mCi] 131I should receive at least verbal instructions). Third, a record documenting justification of the assigned OFs and the effective half-lives used in exposure calculations must be maintained for 3 y after the date of patient release. Fourth, state law may preclude the use of OF in determining release criteria. State-imposed nuclear regulations should be reviewed before implementation of release criteria based on OF.
CONCLUSION
The use of the equations and tables can facilitate calculation of the 131I Qmax that may be administered to an outpatient. Patient living conditions determine the most appropriate OF to be used for dose calculation. Temporary modification of a patient's lifestyle may allow reassignment of OF. Hence, the willingness and ability of the patient to temporarily modify lifestyle may allow the prescribing physician more latitude in dose selection while maintaining the outpatient setting. The use of appropriately modified OFs with the equations and tables will justify the safe use of appropriate high-dose 131I therapy in most outpatients.
APPENDIX A
Maximum Dosages of 131I for Outpatients
-
Determine or estimate the fractional uptake of 131I (may be assumed to be 0.05 in most cases).
-
Establish the duration of the period of constrained activity (is generally assigned a value of 2 d).
-
Immediately after dose administration, 3 distinct time periods are considered: the preequilibrium period (first 8 h after dose administration), the period of constrained activity (duration of physician-imposed constrained activity after preequilibrium period), and the following period of unconstrained activity.
-
Assign OFp, OFc, and OFuc, or the fraction of time that the person most with the patient is actually with the patient for each of the 3 periods.
-
Find the maximum dose in Table 1 as a function of OFp, OFc, and OFuc, where the fractional uptake is less than or equal to 0.05 and the period of constrained activity is 2 d, or find the maximum dose using Tables 2 and 3 and Equation 4.
APPENDIX B
Glossary
- U:
- percentage thyroid 131I uptake.
- OF:
- occupancy factor.
- OFp:
- occupancy factor during the preequilibrium period.
- OFc:
- occupancy factor during the constrained period.
- OFuc:
- occupancy factor during the unconstrained period.
- C:
- duration of period of constrained activity (days).
- Q:
- activity (millicuries).
- Qmax:
- maximum allowable dose that can be given to an outpatient.
- Q0:
- initial dose.
- T:
- effective half-life.
- Tp:
- effective half-life, preequilibrium component.
- Tth:
- effective half-life, thyroidal component.
- Te:
- effective half-life, extrathyroidal component.
- E:
- unintended exposure received by an individual secondary to exposure to the patient.
- Ep:
- unintended exposure (millisieverts), preequilibrium component.
- Eth:
- unintended exposure (millisieverts), thyroidal component.
- Ee:
- unintended exposure (millisieverts), extrathyroidal component.
- T:
- time of exposure (days).
- R:
- distance from source (meters).
- Γ:
- exposure rate constant, (0.0528 mSv × m2)/(MCi × d), for 131I.
Acknowledgments
The authors thank Stephen McGuire, PhD, Richard McKinley, and David Everhardt for review and comments and Diane Voelker for assistance with manuscript preparation.
Footnotes
Received Sep. 13, 1999; revision accepted Feb. 1, 2000.
For correspondence or reprints contact: Leonard R. Coover, MD, Hamot Medical Center, 201 State St., Erie, PA 16550.









