Visual Abstract
Abstract
68Ga-labeled fibroblast activation protein inhibitor (68Ga-FAPI) PET/CT has demonstrated promising clinical results, with a higher SUVmax and tumor-to-background ratio (TBR) in breast cancer (BC) patients than 18F-FDG PET/CT. Here, we aimed to evaluate the suitability of 68Ga-FAPI PET/CT for the early and late prediction of the pathologic response to neoadjuvant chemotherapy (NAC) in BC. Methods: Twenty-two consecutive patients with newly diagnosed BC and an indication for NAC were prospectively included. All patients underwent standard chemotherapy and 68Ga-FAPI PET/CT at baseline, after 2 cycles of NAC (PET2), and 1 wk before surgery (PET3). SUVmax was measured in the primary tumor region and positive regional lymph nodes. The expression of fibroblast activation protein in the primary lesion was analyzed by immunohistochemistry. Results: Seven patients (31.8%) achieved a pathologic complete response (pCR), and 15 (68.2%) had residual tumors. Thirteen patients (59.1%) showed concentric withdrawal of the primary tumor, and 9 (40.9%) showed diffuse withdrawal. Between PET2 and PET3, the ΔSUVmax of the primary tumor (R2 = 0.822; P = 0.001) and metastatic lymph nodes (R2 = 0.645; P = 0.002) were significantly correlated. The absolute values of SUVmax and TBR at PET2 and PET3 were lower in patients with pCR than in those without pCR (P < 0.05). Moreover, a larger ΔSUVmax at any time point was strongly associated with pCR (P < 0.05). Similar downward trends in SUVmax, TBR, and ΔSUVmax were observed in the pattern of primary tumor reduction. For predicting pCR, the optimal cutoff values for ΔSUVmax after 2 chemotherapy cycles, ΔSUVmax before surgery, TBR after 2 chemotherapy cycles, and TBR before surgery of the primary tumor were 3.4 (area under the curve [AUC], 0.890), 1.1 (AUC, 0.978), −63.8% (AUC, 0.879), −90.8% (AUC, 0.978), 7.6 (AUC, 0.848), and 1.4 (AUC, 0.971), respectively. Immunohistochemistry showed that the SUVmax and TBR of 68Ga-FAPI PET/CT were positively correlated with fibroblast activation protein expression (P < 0.001 for both). Conclusion: Assessment of early changes in 68Ga-FAPI uptake during NAC by 68Ga-FAPI PET/CT can predict pCR and primary tumor concentric withdrawal in BC patients. 68Ga-FAPI PET/CT has great potential for the early and late prediction of the pathologic response to NAC in BC.
- fibroblast activation protein inhibitor
- 68Ga-FAPI
- neoadjuvant chemotherapy
- pathologic response
- breast cancer
Neoadjuvant therapy has become a very important component of current comprehensive treatments for breast cancer (BC). Neoadjuvant chemotherapy (NAC) can reduce the tumor–node–metastasis stage of BC in most patients to allow surgery or breast preservation to take place; it can also be used to evaluate drug sensitivity in vivo to improve survival (1). A pathologic complete response (pCR) can be used as an alternative research endpoint indicating prognosis in clinical trials of neoadjuvant drugs for BC (2). Whether pCR is achieved is an important basis for adjusting the follow-up adjuvant treatment scheme in BC patients (3,4).
At present, there is no recognized evaluation method to accurately predict whether pCR can be reached after the full course of treatment at an early stage of neoadjuvant treatment (2 or 4 courses of treatment). Methods involving PET/CT, breast MRI, genomics, and Imageomics (Imageomics Institute) are still in clinical trials. Because the metabolic decline in tumors may occur earlier than the anatomic changes caused by NAC, 18F-FDG PET/CT could be an option for assessing changes in tumor viability to monitor the response to NAC (5,6).
In recent years, 68Ga-labeled fibroblast activation protein inhibitor (68Ga-FAPI) PET/CT has demonstrated a higher SUVmax and tumor-to-background ratio (TBR) than 18F-FDG PET/CT in BC (7–9). Furthermore, in the study by Backhaus et al. (10), 68Ga-FAPI PET/MRI showed promising diagnostic performance for response assessment after NAC for BC. Therefore, the use of 68Ga-FAPI PET/CT to predict the efficacy of NAC for BC would be desirable.
In this prospective study, we first focused on whether 68Ga-FAPI PET/CT is useful in predicting pCR in BC patients undergoing NAC. The secondary objectives were to evaluate the changes in 68Ga-FAPI PET/CT in the primary tumor and lymph nodes of BC and then assess the withdrawal pattern of BC after NAC. This information is very important in the selection of breast-conserving surgery (BCR) after NAC for BC.
MATERIALS AND METHODS
Patients
This prospective study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Approval No. MRCTA; ECFAH of FMU [2019] 293) and as part of an umbrella study registered online at ClinicalTrials.gov (NCT04499365). A total of 25 patients who had newly diagnosed BC confirmed by biopsy and who received NAC were consecutively recruited from March 2021 to July 2022. All subjects signed a written informed consent form.
The key eligibility criteria were as follows: adult patients (18–70 y old); patients who had primary BC confirmed by pathology and who were treatment-naive before 68Ga-FAPI PET/CT scans; and patients who met the indications of NAC for BC, completed the full course of NAC, and finally received surgical treatment. Patients who met all of these criteria were included in the study. The exclusion criteria were as follows: patients who were unwilling to undergo a 68Ga-FAPI PET/CT scan; patients who could not tolerate chemotherapy or surgery; patients who had any other malignant tumors or who had received any form of antitumor treatment in the past; and patients with evidence of distant metastasis after examination. Three patients were excluded from the study on the basis of these criteria.
All patients underwent 68Ga-FAPI PET/CT at baseline (PET1), after 2 cycles of NAC (PET2), and 1 wk before surgery (PET3).
Examination Procedure for 68Ga-FAPI PET/CT
All imaging was performed on dedicated PET/CT scanners (Biograph mCT 64; Siemens Healthcare). The subject was injected with 68Ga-FAPI-04 at 1.8–2.2 MBq/kg intravenously into the elbow. After quiet resting for 30 min, a low-dose CT scan without contrast was performed from the top of the skull to the midthigh. CT scanning was performed with the following parameters: 110 kV, 120 mAs, and 3-mm layer thickness. A PET scan was performed immediately after the CT scan. The 3-dimensional mode (matrix, 200 × 200) acquisition method was performed with 3-min/bed positions. Data were obtained by the 3-dimensional row-action maximum-likelihood algorithm method to provide attenuation correction images.
Then, images were analyzed independently on a Syngo MultiModality Workplace (Siemens) by 2 experienced nuclear medicine physicians. Any disagreement was resolved through discussion with a third experienced physician and by integrating other imaging data. If the activity of the primary tumor or regional lymph node exceeded that of the adjacent background tissue, then it was marked as positive. The SUVmax was obtained in the region drawn around the primary tumor and positive regional lymph nodes and was calculated for semiquantitative analysis. The TBR was calculated by dividing the lesion SUVmax by the SUVmean of the contralateral normal breast tissue. Compared with the baseline value (SUVmax1), the percentages of ΔSUVmax in the region of interest after 2 chemotherapy cycles (SUVmax2) and before surgery (SUVmax3) were calculated as follows:
 and
and
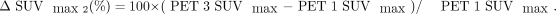
NAC Protocols
All patients were confirmed to have BC by a core-needle biopsy. Immunohistochemical staining of estrogen receptor, progesterone receptor, Ki-67, FAP, and human epidermal growth factor receptor (HER2) was performed routinely (supplemental material; supplemental materials are available at http://jnm.snmjournals.org).
Referring to previous studies (11,12), the BC molecular subtypes were classified as follows: luminal A–like tumors, luminal B/HER2-negative–like tumors, luminal B/HER2-positive–like tumors, HER2-positive–like tumors, and triple-negative–like tumors. The NAC regimen used was the standard recommended by the guidelines (12); details are included in the supplemental material.
Surgery and Pathology Assessment
Mastectomy was performed in 16 patients, and BCR was performed in 6 patients. According to the initial state of the axillary lymph nodes, 3 patients without clinically suspect signs underwent axillary sentinel lymph node dissection, and 19 patients underwent axillary lymph node dissection.
pCR was defined as the primary breast tumor lacking invasive cancer and the regional lymph node showing a negative result (2). The presence of cancer in situ components was allowed. For non-pCR patients, the residual cancer burden (RCB) scoring system (13) was further used to evaluate the degree of tumor remission. The pattern of tumor reduction (concentric or diffuse) was also observed.
Statistical Analysis
Statistical analysis was performed using the IBM SPSS 25.0 package (SPSS 25.0 for MAC; IBM SPSS). An independent Student t test was used for continuous variables, and the Pearson χ2 test or Fisher exact test was used for categoric variables. Receiver operating characteristic curves and the area under the curve (AUC) were determined for regression models according to 68Ga-FAPI uptake. The Youden index was used to estimate the optimal cutoff value. Diagnostic agreements between 68Ga-FAPI uptake and pCR or primary tumor shrinkage was assessed by sensitivity, specificity, positive predictive value, negative predictive value, and the Cohen κ-index. All statistical analyses were performed using 2-sided tests, and a P value of less than 0.05 was considered the threshold for statistical significance.
RESULTS
Patient Characteristics
According to the inclusion criteria, a total of 25 patients were enrolled; of these, 3 patients were excluded because they met at least 1 of the exclusion criteria. The clinicopathologic data for the 22 consecutive patients finally enrolled are detailed in Table 1.
Overall Characteristics of Breast Cancer Patients
Correlation Between PET2 and PET3
The median ± SD ΔSUVmax of the primary tumor at PET2 was –32.4% ± 48.1% (range, –89.0% to 112.1%), and that at PET3 was –73.9% ± 20.6% (range, –96.8% to –35.4%). In 20 patients with lymph node metastasis, the median ΔSUVmax of metastatic lymph nodes (MLNs) at PET2 and PET3 were –54.0% ± 39.0% (range, –92.7% to 70.9%) and –85.0% ± 14.9% (range, –100.0% to –52.6%), respectively. Between PET2 and PET3, there were significant correlations of the ΔSUVmax of the primary tumor (R2 = 0.822 [P = 0.001]) and MLNs (R2 = 0.645 [P = 0.002]) (Fig. 1).
Correlation between relative ΔSUVmax of primary tumor (A) and metastatic lymph node (B) at PET2 and PET3.
Relationship Between 68Ga-FAPI Uptake Changes and pCR
Of the 22 patients, 7 (31.8%) achieved pCR, and 15 (68.2%) showed residual tumor in the final pathology findings. According to the RCB scoring system, there were 7 (31.8%) with RCB0, 1 (4.5%) with RCB1, 6 (27.3%) with RCB2, and 8 (36.4%) with RCB3.
For the primary tumor, the SUVmax1 at baseline was not associated with pCR. However, after NAC, most patients showed various degrees of decrease in SUVmax2, SUVmax3, TBR after 2 chemotherapy cycles (TBR2), and TBR before surgery (TBR3), with patients with pCR showing a more significant decrease. As Table 2 shows, the absolute values of SUVmax2, SUVmax3, TBR2, and TBR3 in patients with pCR were lower than those in patients without pCR (4.7 ± 2.9 vs. 12.7 ± 8.4 [P = 0.024], 1.1 ± 0.3 vs. 5.2 ± 3.9 [P = 0.001], 4.8 ± 4.0 vs. 11.9 ± 7.4 [P = 0.027], and 1.0 ± 0.3 vs. 4.7 ± 3.6 [P = 0.001], respectively). Moreover, the larger ΔSUVmax between PET1, PET2, and PET3 were strongly associated with the pCR rate (Fig. 2). The ΔSUVmax1 and ΔSUVmax2 in the pCR and non-pCR groups were −70.5% ± 25.6% versus –14.6% ± 46.2% (P = 0.008) and −93.5% ± 2.0% versus –64.8% ± 18.8% (P = 0.001), respectively. Similar downward trends in SUVmax and ΔSUVmax were also observed in MLNs (Figs. 2 and 3). However, when the 68Ga-FAPI uptake of MLNs alone was considered, only SUVmax3 and ΔSUVmax2 could better predict pCR than SUVmax2 and ΔSUVmax1 (P = 0.001).
Correlations Between 68Ga-FAPI PET/CT Parameters and Pathologic Response
Box plots of ΔSUVmax and pathologic response (pCR vs. non-pCR) at PET2 and PET3. *P < 0.05. **P < 0.01. ***P < 0.001.
Changes in SUVmax of primary tumor (A) and metastatic lymph node (B) according to pathologic response (pCR vs. non-pCR) at PET1, PET2, and PET3.
Relationship Between 68Ga-FAPI Uptake Changes and Primary Tumor Withdrawal
With regard to the pattern of primary tumor reduction, there were 13 patients (59.1%) with concentric withdrawal and 9 (40.9%) with diffuse withdrawal. Of the 6 BCR patients, 2 underwent extensive resection of the incisal margin because of diffuse withdrawal in the postoperative pathologic evaluation.
Compared with those in patients with diffuse withdrawal, the SUVmax2, SUVmax3, TBR2, and TBR3 of the primary tumor in patients with concentric withdrawal were lower, and the decline in ΔSUVmax1 and ΔSUVmax2 was greater (all Ps < 0.05). Regarding the 68Ga-FAPI uptake changes in MLNs, there was little correlation with the regression pattern of the tumor (Fig. 4; Table 2).
Changes in SUVmax of primary tumor according to pathologic response (concentric withdrawal vs. diffuse withdrawal) at PET1, PET2, and PET3.
Prediction of pCR and Concentric Withdrawal According to 68Ga-FAPI Uptake
In predicting pCR, 3.4 (AUC, 0.890), 1.1 (AUC, 0.978), 7.6 (AUC, 0.848), and 1.4 (AUC, 0.971) were the optimal cutoff values for SUVmax2, SUVmax3, TBR2, and TBR3 of the primary tumor, respectively. The optimal cutoff values for ΔSUVmax1 and ΔSUVmax2 were −63.8% (AUC, 0.879) and −90.8% (AUC, 0.978), respectively. The optimal cutoff values for SUVmax3 and ΔSUVmax2 of the MLNs were 0.5 (AUC, 0.901) and −88.8% (AUC, 0.945), respectively.
In predicting concentric withdrawal of the primary tumor, the optimal cutoff values for SUVmax2, SUVmax3, TBR2, and TBR3 of the primary tumor were 14.3 (AUC, 0.824), 3.1 (AUC, 0.857), 9.5 (AUC, 0.821), and 2.5 (AUC, 0.872), respectively. Those for ΔSUVmax1 and ΔSUVmax2 were −24.0% (AUC, 0.824) and −90.8% (AUC, 0.824), respectively.
These selected critical values for primary tumor uptake were further used as cutoff values to evaluate the diagnostic agreement (Table 3). The SUVmax2 and TBR3 of the primary tumor criteria showed the highest sensitivity in predicting pCR (100% for all). The SUVmax3 and ΔSUVmax2 of the primary tumor criteria had the highest specificity in predicting pCR (100% for all). The SUVmax3, TBR3, and ΔSUVmax2 of the primary tumor criteria had the highest κ-index in predicting pCR (0.899).
Diagnostic Agreement of 68Ga-FAPI Uptake Cutoff Values with Favorable Pathologic Response of Primary Tumor
In predicting primary tumor concentric withdrawal, the ΔSUVmax2 of the primary tumor criteria had the highest sensitivity (100%). The SUVmax2 of the primary tumor criteria had the highest specificity (100%). The SUVmax3 of the primary tumor criteria had the highest κ-index (0.723).
Association Between FAP Immunohistochemistry and 68Ga-FAPI Uptake
We performed immunohistochemical staining for FAP on both biopsy specimens and postoperative specimens of the primary tumor. The SUVmax and TBR of 68Ga-FAPI PET/CT were positively correlated with FAP expression in the stroma of the BC lesions (r = 0.886 [P < 0.001] and r = 0.556 [P < 0.001], respectively). Figures 5 and 6 show images of 2 patients as representative examples.
Representative 68Ga-FAPI PET/CT images of 48-y-old woman who had left invasive ductal carcinoma and who achieved pCR. (A) Baseline 68Ga-FAPI PET/CT maximum-intensity-projection (MIP) image demonstrated primary lesion (red arrowhead; SUVmax, 26.72) and 68Ga-FAPI–avid lymph node in left axilla (red arrows). (B) 68Ga-FAPI PET/CT MIP image after 2 cycles of NAC showed that primary lesion (red arrowhead; SUVmax, 8.87) and axillary lymph nodes (red arrow) were reduced in size and radioactivity uptake. (C and D) Representative images of immunohistochemical staining for FAP before NAC showed strong FAP-positive staining in stromal cells (C), which was significantly decreased on tumor bed after 6 cycles of NAC (D).
Representative 68Ga-FAPI PET/CT images of 46-y-old woman who had left invasive ductal carcinoma and who did not achieve pCR. (A) Baseline 68Ga-FAPI PET/CT maximum-intensity-projection (MIP) image demonstrated primary lesion (red arrowhead; SUVmax, 11.62). (B) 68Ga-FAPI PET/CT MIP image after 2 cycles of NAC showed that primary lesion (red arrowhead; SUVmax, 10.59) was smaller and that radioactivity uptake was slightly lower than before. (C and D) Representative images of immunohistochemical staining for FAP before NAC showed strong FAP-positive staining in stromal cells (C), but no obvious change was observed on tumor bed after 6 cycles of NAC (D).
DISCUSSION
In the present study, we demonstrated the clinical practicality of using 68Ga-FAPI uptake based on preprocessed 68Ga-FAPI PET/CT for predicting the pathologic response to NAC treatment in BC patients.
Under NAC, the lower SUVmax2, SUVmax3, TBR2, and TBR3 as seen on 68Ga-FAPI PET/CT and the larger ΔSUVmax1 and ΔSUVmax2 were strongly associated with a higher pCR rate. Moreover, 68Ga-FAPI PET/CT had a high value in the early response evaluation. Patients with no response at PET2 had a higher rate of obtaining a score of RCB2 or RCB3 in the final pathologic evaluation. Our research showed that 68Ga-FAPI PET/CT can rapidly provide feedback on tumor changes through 68Ga-FAPI uptake after 2 cycles of chemotherapy. Accurate evaluation can lead clinicians to adjust their chemotherapy regimen as soon as possible to avoid ineffective chemotherapy for patients who have not achieved pCR or who show poor therapeutic effects.
In recent years, 18F-FDG PET/CT has been optionally used to monitor the responses to NAC in BC patients (5,14). In previous metaanalyses for 18F-FDG PET/CT, it was found that the predictive value of SUVmax has high sensitivity but relatively low specificity in predicting pCR (15,16). There are 2 obvious limitations in the clinical application of 18F-FDG PET/CT for the early prediction of a pathologic response. First, 18F-FDG PET has limited sensitivity in detecting small breast lesions, lobular cancer, or low-grade BC (17,18). A previous study showed that ΔSUVmax obtained with 18F-FDG PET can only be evaluated when the TBR is higher than 5 in BC (19). In addition, the patient’s blood glucose concentration, insulin concentration, and level of inflammation may influence 18F-FDG PET/CT parameters (20). During NAC, insulin resistance and blood sugar changes may occur, and myelosuppression can also cause inflammation. These factors also increase the instability of 18F-FDG PET/CT monitoring.
Reportedly, the expression of FAP is enhanced at tumor boundaries and invasive frontiers, such as tumor cell protrusions, under the microscope (21). Ristau et al. (22) reported the complementary role of 68Ga-FAPI PET/CT in accurately depicting the volume of primary tumors. Their results indicated that 68Ga-FAPI PET/CT examination is less affected by the patient’s blood sugar and inflammatory factors. In addition, in our previous research, 68Ga-FAPI PET/CT showed a higher SUVmax and TBR than 18F-FDG PET/CT in primary tumors, and it also showed advantages in identifying regional lymph node metastasis (9). In addition, previous studies found no differences in 68Ga-FAPI uptake based on histopathologic features such as immunohistochemistry and grading (7,8). These factors all facilitate precise evaluation using 68Ga-FAPI PET/CT. In the study by Backhaus et al. (10), integrated 68Ga-FAPI PET/MRI was used to classify the response in BC correctly on the basis of readers’ visual assessment or TBR. In the present study, 68Ga-FAPI PET/CT measures also showed high accuracy in predicting pCR.
In the present study, we also explored the ability of 68Ga-FAPI PET/CT to predict tumor withdrawal patterns after NAC. Downstaging primary tumors to allow for BCR is one of the goals of NAC. A metaanalysis of the Early Breast Cancer Trialists’ Collaborative Group showed that NAC can increase the probability of BCR, but it can also lead to an increase in local recurrence rate (23). How to accurately evaluate residual tumors after NAC is the crux of clinical treatment. Currently, MRI is the most commonly used method for evaluating tumor withdrawal after NAC. In the present study, the parameters of FAP uptake better evaluated the tumor withdrawal pattern, especially when 68Ga-FAPI PET/CT was done 1 wk before surgery.
The present study has some limitations that must be considered. First, this pilot prospective study was conducted in a single institution with a limited sample size. Further multiinstitutional studies with large samples are needed to validate our results. Second, we did not explore those BC patients separately on the basis of molecular subtypes. BC is a heterogeneous disease, and different molecular subtypes and chemotherapy regimens can also affect the reduction in SUVs (24,25). In the present study, we did not include patients with luminal A subtype BC. A larger patient population is needed in further trials to clarify this point. The applicability of 68Ga-FAPI PET/CT to predict the early pathologic response of different subgroups of BC is the focus of our follow-up study. Third, in the present study, neoadjuvant treatment only included chemotherapy (with or without HER2-targeted therapy). Therefore, the ability of 68Ga-FAPI PET/CT to assess the response to other neoadjuvant treatments, such as hormonal therapy or immunotherapy, cannot be inferred.
CONCLUSION
The present study suggests that during NAC, early changes in 68Ga-FAPI uptake (SUVmax, TBR, and ΔSUVmax) assessed by 68Ga-FAPI PET/CT are powerful parameters for predicting pCR in BC patients. 68Ga-FAPI PET/CT parameters can rapidly provide feedback on tumor changes after only 2 cycles of chemotherapy. For predicting primary tumor concentric withdrawal and determining the appropriateness of BCR, imaging at PET3 is further recommended. Future studies should consider cancer subtypes and chemotherapy regimens and further validate 68Ga-FAPI PET/CT as an early response indicator to support its use as a better clinical tool for guiding patient treatment.
DISCLOSURE
This research was funded by the Fujian Provincial Health Technology Project (2022CXA020) and the Natural Science Foundation of Fujian Province (2021J01720). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can 68Ga-FAPI PET/CT predict the pathologic response to NAC in BC patients?
PERTINENT FINDINGS: Assessment of early changes in 68Ga-FAPI uptake (SUVmax, TBR, and ΔSUVmax) during NAC by 68Ga-FAPI PET/CT can predict pCR and primary tumor concentric withdrawal in BC patients.
IMPLICATIONS FOR PATIENT CARE: 68Ga-FAPI PET/CT has great potential for the early prediction of a pathologic response to NAC in BC, supporting its use as a better clinical tool for guiding patient treatment.
Footnotes
↵* Contributed equally to this work.
Published online Nov. 2, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications. License: https://creativecommons.org/licenses/by/4.0/. Details: http://jnm.snmjournals.org/site/misc/permission.xhtml.
REFERENCES
- Received for publication May 26, 2023.
- Revision received September 27, 2023.