Visual Abstract
Abstract
Neoadjuvant therapy in patients with locally advanced rectal cancer (LARC) has achieved good pathologic complete response (pCR) rates, potentially eliminating the need for surgical intervention. This study investigated preoperative methods for predicting pCR after neoadjuvant short-course radiotherapy (SCRT) combined with immunochemotherapy. Methods: Treatment-naïve patients with histologically confirmed LARC were enrolled from February 2023 to July 2023. Before surgery, the patients received neoadjuvant SCRT followed by 2 cycles of capecitabine and oxaliplatin plus camrelizumab. 68Ga-labeled fibroblast activation protein inhibitor ([68Ga]Ga-FAPI-04) PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI were performed before treatment initiation and before surgery in each patient. PET and MRI features and the size and number of lesions were also collected from each scan. Each parameter’s sensitivity, specificity, and diagnostic cutoff were derived via receiver-operating-characteristic curve analysis. Results: Twenty eligible patients (13 men, 7 women; mean age, 60.2 y) were enrolled and completed the entire trial, and all patients had proficient mismatch repair or microsatellite-stable LARC. A postoperative pCR was achieved in 9 patients (45.0%). In the visual evaluation, both [68Ga]Ga-FAPI-04 PET/MRI and [18F]FDG PET/CT were limited to forecasting pCR. Contrast-enhanced MRI had a low sensitivity of 55.56% to predict pCR. In the quantitative evaluation, [68Ga]Ga-FAPI-04 change in SULpeak percentage, where SULpeak is SUVpeak standardized by lean body mass, had the largest area under the curve (0.929) with high specificity (sensitivity, 77.78%; specificity, 100.0%; cutoff, 63.92%). Conclusion: [68Ga]Ga-FAPI-04 PET/MRI is a promising imaging modality for predicting pCR after SCRT combined with immunochemotherapy. The SULpeak decrease exceeding 63.92% may provide valuable guidance in selecting patients who can forgo surgery after neoadjuvant therapy.
Colorectal cancer poses a significant public health burden, accounting for approximately 8% of all new cancer cases (1). In particular, locally advanced rectal cancer (LARC; T3–4 or N+) has been challenging in terms of treatment and organ preservation because of its complex anatomic structure and high rates of postoperative complications and recurrence (2). Preoperative neoadjuvant therapy has displayed significant efficacy in inducing substantial tumor shrinkage and downstaging and in achieving a pathologic complete response (pCR). pCR refers to the absence of evidence of viable tumor cells, suggesting that patients may forgo surgical intervention and avoid postoperative complications (3). These patients may benefit from a rectal preservation strategy, resulting in a high quality of life (4). Immune checkpoint inhibitor therapy activates cytotoxic T lymphocytes in patients, significantly increasing the pCR rate (5). Recently, we conducted a clinical trial using a novel preoperative short-course radiotherapy (SCRT) regimen and subsequent immunochemotherapy in patients with LARC and recorded a favorable pCR rate (42/105, 40.0%) for proficient mismatch repair or microsatellite-stable tumors (6). This treatment approach is expected to provide more opportunities to delay or forgo surgery among patients with middle-to-low LARC and for organ function to be preserved in the future.
Given the escalating rate of pCR in treating LARC, it is imperative to seek an effective preoperative method for predicting candidates likely to experience pCR and avoid surgical intervention (7). In the preoperative assessment, digital rectal examination, endoscopic ultrasound, and MRI serve as the cornerstones of pCR identification (8,9). However, in evaluating emerging neoadjuvant immunotherapy, these examination modalities are limited to differentiating necrotic tumor tissue from residual lesions (10). In terms of [18F]FDG PET/CT, immunotherapy can lead to an abnormal [18F]FDG accumulation because of T-cell infiltration in the tumor, which might limit the detection of residual lesions (11). Fibroblast activation protein (FAP) expression is notably upregulated in cancer-associated fibroblasts within various tumor stroma, contrasting with its scarcity in normal tissues (12). Recent studies have also highlighted that 68Ga-labeled FAP inhibitor ([68Ga]Ga-FAPI-04) exhibits negligible uptake in the intestinal wall and is superior to [18F]FDG in diagnosing patients with colorectal cancer (13,14).
We conducted a prospective trial using the therapeutic schedule of preoperative SCRT followed by 2-cycle immunochemotherapy in patients with LARC. [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI were performed before treatment initiation and before surgery for each patient for the initial and preoperative assessment, respectively. This study aimed to seek the value of those modalities in predicting pCR in patients before surgery.
MATERIALS AND METHODS
Study Patients
The institutional review board of our hospital approved this prospective and single-center trial (ClinicalTrials.gov registration number NCT05999227) performed from February 2023 to July 2023. Every patient gave written informed consent before undergoing [68Ga]Ga-FAPI-04 PET/MRI and [18F]FDG PET/CT. The key eligibility criteria were as follows: histologically confirmed treatment-naïve T3–4N0M0 or T1–4N+M0 rectal adenocarcinoma; no severe hematologic, cardiac, pulmonary, hepatic, or renal functional abnormalities or immunodeficiency diseases; and an Eastern Cooperative Oncology Group performance status of 0–1. The exclusion criteria were as follows: prior anti–programmed death ligand 1 or anti–programmed cell death 1 antibody treatment; a history of pelvic radiation; the presence of autoimmune disease; hypersensitivity to any monoclonal antibodies; a history of interstitial lung disease; and active and uncontrolled infection.
Procedures
After enrollment, [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI were performed within an interval of less than 1 wk for each patient for disease assessment (Fig. 1). Then, the patients received SCRT (5 Gy/d for 5 d), followed 1 wk later by 2 subsequent 21-d cycles of neoadjuvant immunochemotherapy (oxaliplatin, 130 mg/m2 intravenously on day 1; capecitabine, 1,000 mg/m2 orally twice daily on days 1–14; and camrelizumab, 200 mg/m2 by intravenous drip on day 1). Three weeks after the second cycle, the 3 imaging modalities were repeated for preoperative evaluation, followed by radical surgery within 1 wk. All resected specimens (including the whole tumor bed, sampled lymph nodes, and surrounding tissues) were sampled and cross-sectioned consecutively. pCR was defined as the absence of any remaining viable cancer cells in the resected primary tumor specimen and all sampled regional lymph nodes (ypT0N0).
Procedures of clinical trial. CAPOX = capecitabine plus oxaliplatin.
[68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and Contrast-Enhanced MRI Acquisition
A 1.8–2.2 MBq/kg dose of [68Ga]Ga-FAPI-04 was administered intravenously. After approximately 30 min, the patients underwent hybrid time-of-flight PET/MRI. The MRI protocols for PET/MRI included T1-weighted, T2-weighted, and diffusion-weighted imaging. [18F]FDG imaging was performed on a PET/CT scanner (Discovery VCT; GE HealthCare) approximately 60 min after [18F]FDG (3.7–5.5 MBq/kg) administration. Contrast-enhanced MRI was performed with 8 phases (1 precontrast and 7 postcontrast phases) using a modified Dixon sequence on a 3.0-T MRI unit (Philips Ingenia).
Image Interpretation
PET images were analyzed using a PET volume computer-assisted reading software (GE HealthCare). A volume of interest around the lesion was autocontoured and segmented. Then, the volume of interest was further adjusted by 2 nuclear medicine physicians to ensure that it contained all the PET-positive areas and excluded negative normal tissue. The PET parameters, including SUV, SUV standardized by lean body mass (SUL), metabolic tumor volume (MTV), and FAP-expressing tumor volume (FTV), were automatically obtained. Total lesion glycolysis (TLG) and total lesion FAP expression (TLF) were calculated as the product of SUVmean and MTV or FTV. The tumor-to-liver ratio (TLR) was calculated as the SULpeak of the targeted lesion to the SULmean of the right liver lobe. The formula for ΔTLR% was as follows: ΔTLR% = (TLR of pretherapy − TLR of posttherapy)/TLR of pretherapy × 100%. The values for ΔSUVmax%, ΔSULpeak%, ΔMTV%, ΔFTV%, ΔTLG%, and ΔTLF% were similarly calculated.
The MRI evaluation included T- and N-stage measurements, extramural venous invasion, mesorectal fascia invasion, and tumor location (15). The reader criteria for contrast-enhanced MRI distinguishing preoperative yT0–1 from yT2–4 tumors and positive lymph nodes are shown in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org) (15,16).
Statistical Analysis
SPSS 22.0 (IBM Inc.) was used for data processing. Continuous variables are expressed as means ± SDs. Categoric variables are expressed as numbers and percentages. The number of positive lesions revealed by the 3 imaging modalities was compared using the χ2 test or Fisher–Freeman–Halton exact test. The Student t test, Welch t test, or Mann–Whitney U test was used to test differences in quantitative parameters. The sensitivity and specificity of each parameter were determined using receiver-operating-characteristic curve analysis to predict pCR. Two-tailed P values of less than 0.05 were considered statistically significant.
RESULTS
Patient Characteristics and Compliance
In total, 27 patients were screened, and 7 patients were excluded for the reasons given in Figure 2. Patient baseline and disease characteristics are summarized in Table 1. Most patients (80.0%, 16/20) had at least 1 high-risk factor, including cT4 disease (10.0%, 2/20), cN2 disease (30.0%, 6/20), extramural vascular invasion (45.0%, 9/20), and tumors within 5 cm of the anal verge (35.0%, 7/20). All patients had proficient mismatch repair or microsatellite-stable tumors.
Trial profile.
Patient Characteristics (n = 20)
Surgery and Pathology
The pCR (ypT0N0, tumor regression grade 0) rate was 45.0% (9/20), and negative nodes (ypN0) were reported in 16 patients. In terms of non-pCR patients, the tumor regression grade 1, 2, and 3 rates were 20.0% (ypT2), 30.0% (4 ypT3, 2 ypT2), and 5.0% (ypT3), respectively. No patient with ypT1 was identified, suggesting that contrast-enhanced MRI can be used to distinguish between ypT0 and ypT2–3 in this group of patients. Nine positive nodes were examined in surgery, and the ypN1a, ypN1b, and ypN2a rates were 10.0%, 5.0%, and 5.0%, respectively. After radical surgery, 2 patients developed postoperative infection, and 1 patient experienced bleeding. No other postoperative complications occurred, and there were no treatment-related deaths.
Relationship Between Clinical Characteristics and pCR
To identify the factors influencing pathologic response, clinical characteristics (Table 2), including age, sex, clinical T/N category, disease stage at baseline, and laboratory parameters at baseline and before surgery, were analyzed. The neutrophil percentage at baseline and preoperative interleukin-4 levels were higher in patients with pCR than in non-pCR patients.
Clinical Characteristics of Patients According to Pathologic Response
Performance of [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and Contrast-Enhanced MRI
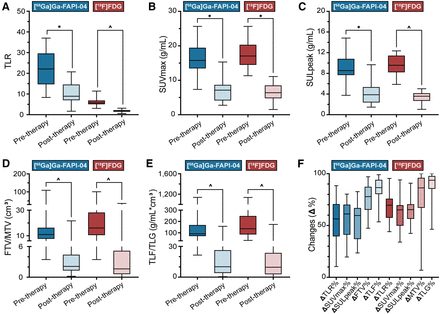
[68Ga]Ga-FAPI-04 PET/MRI could visually detect the primary lesion in all patients (20/20) at baseline. However, [68Ga]Ga-FAPI-04 PET/MRI remained positive in all patients after therapy, limiting its ability to forecast pCR (e.g., patient 4; Fig. 3). In quantitative analysis (Table 3), none of the [68Ga]Ga-FAPI-04 PET parameters had a relationship with pCR in pretherapy scanning. These PET parameters dramatically decreased (Fig. 4) and became significantly correlated with pCR after therapy (P < 0.05).
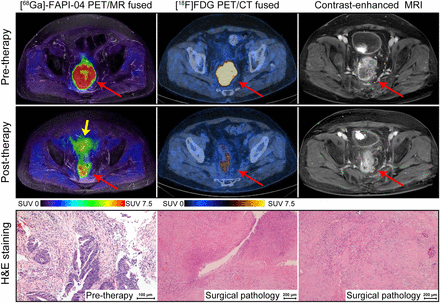
A 70-y-old man sought medical attention for 2-mo history of diarrhea. Colonoscopy revealed neoplasm in upper-middle rectum, and further pathology was indicative of rectal adenocarcinoma. At baseline, [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI revealed avid tracer accumulation and asymmetric wall thickening (red arrows) in upper-middle rectum. Focal uptake of [68Ga]Ga-FAPI-04 and [18F]FDG was detected after neoadjuvant SCRT combined with immunochemotherapy. Contrast-enhanced MRI showed nodular enhancement exceeding outermost edge of rectal wall. However, further surgical pathology identified no residual tumor cells (pCR), but chronic ulcer with hyperplasia of granulation tissue was noted. Yellow arrow points to bladder. H&E = hematoxylin and eosin.
PET Characteristics of Patients According to Pathologic Response
Changes in PET parameters in pretherapy and posttherapy scans: TLR (A); SUVmax (B); SULpeak (C); FTV/MTV (D); TLF/TLG (E); and ΔTLR%, ΔSUVmax%, ΔSULpeak%, ΔFTV/MTV%, and ΔTLF/TLG% (F) from [68Ga]Ga-FAPI-04 PET/MRI and [18F]FDG PET/CT. *P < 0.0001. ^P < 0.0001. ^P values were calculated using Mann–Whitney U test.
The visual evaluation of [18F]FDG PET/CT imaging was also positive before and after treatment. In quantitative analysis, MTV derived from the posttherapy scan was significantly lower in the pCR group than in the non-pCR group, whereas ΔMTV% and ΔTLG% were significantly higher in the pCR group (P < 0.05).
For contrast-enhanced MRI, the primary lesion could be found in all patients (20/20) at baseline. After therapy, a complete MRI response was detected in 5 of 9 patients, for a sensitivity of 55.56%.
Receiver-Operating-Characteristic Analyses of Imaging and Clinical Parameters for pCR
Receiver-operating-characteristic curve analyses were further applied to identify parameters predictive of pCR (Table 4; Supplemental Fig. 2). Among the [68Ga]Ga-FAPI-04 PET parameters, ΔSULpeak% had the largest area under the curve (0.929, with sensitivity of 77.78%, specificity of 100.0%, and a cutoff of 63.92%). Among [18F]FDG PET parameters, ΔMTV% and ΔTLG% both held the largest area under the curve (0.798, with sensitivity of 88.89% and specificity of 81.82%). As an example, imaging of patient 16 (Fig. 5), with cT3N1aM0 rectal cancer, revealed a remarkable tumor at baseline, and its volume was significantly reduced after 2 cycles of treatment. [68Ga]Ga-FAPI-04 ΔSULpeak% was 61.25% lower than the cutoff in this patient, implying the presence of residual tumor. Contrast-enhanced MRI also exhibited nodular enhancement reaching the outermost edge of the rectal wall. However, [18F]FDG ΔMTV% and ΔTLG% were higher than the cutoffs, suggesting pCR. The surgical histopathologic specimen further revealed tumor regression of grade 2 for the primary tumor, that is, ypT3N0M0.
Diagnostic Performance of Imaging and Clinical Parameters for pCR
A 59-y-old man sought medical attention for 8-y history of hematochezia accompanied by anal bulge and pain for 2 mo. [68Ga]Ga-FAPI-04 PET/MRI and [18F]FDG PET/CT revealed avid tracer accumulation (red arrows) in lower rectum ([68Ga]Ga-FAPI-04: SUVmax of 21.89, SULpeak of 12.36, FTV of 15.12, TLF of 190.33, and TLR of 23.32; [18F]FDG: SUVmax of 14.59, SULpeak of 9.5, MTV of 17.51, TLG of 134.78, and TLR of 5.14) that had markedly decreased ([68Ga]Ga-FAPI-04: SUVmax of 8.21, SULpeak of 4.79, FTV of 6.46, TLF of 28.16, and TLR of 8.71; [18F]FDG: SUVmax of 4.99, SULpeak of 3.04, MTV of 0.49, TLG of 2.37, and TLR of 1.42) after neoadjuvant SCRT combined with immunochemotherapy. Preoperative contrast-enhanced MRI showed nodular enhancement exceeding outermost edge of rectal wall. Further surgical pathology identified presence of residual tumor.
In addition, the efficacy of the 3 imaging modalities in the preoperative N stage was also evaluated. Preoperative [68Ga]Ga-FAPI-04 PET could significantly distinguish positive from negative lymph nodes (Supplemental Table 1; P = 0.001; accuracy of 95.58%, sensitivity of 55.56%, and specificity of 99.04%). [18F]FDG PET and contrast-enhanced MRI had no statistical significance in lymph node identification (Supplemental Fig. 3).
DISCUSSION
In clinical management, achievement of pCR signifies that surgical intervention may be omitted, thereby avoiding postoperative complications and resulting in a higher quality of life for the patient and lower treatment costs (4,17). In this study, we conducted a rigorous, single-center, prospective trial with a unified treatment approach and imaging modalities in patients with tumors at specific stages to seek the available methods for effectively predicting pCR. Certain quantitative PET parameters were able to distinguish patients with pCR. In particular, [68Ga]Ga-FAPI-04 ΔSULpeak% had robust specificity for predicting pCR. When [68Ga]Ga-FAPI-04 ΔSULpeak% exceeds the predefined threshold (63.92%), the patient may achieve pCR after neoadjuvant therapy without surgery. This threshold might be impacted by differences in image acquisition techniques and patient status.
With the new neoadjuvant therapy regimen, patients with proficient mismatch repair or microsatellite-stable LARC had a remarkable pCR rate of 45.0%, similar to the previously reported outcomes (6,18). The high pCR rate suggests that this regimen’s use in proficient mismatch repair or microsatellite-stable LARC confers significant clinical benefits. In the initial assessment, [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI detected the primary lesions. In the posttherapy evaluation, the accumulation of [68Ga]Ga-FAPI-04 and [18F]FDG dramatically declined from before therapy in all patients, indicating the effectiveness of treatment and that the imaging modalities could predict treatment response. However, [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI all display limited predictive value for pCR in terms of visual assessment. [18F]FDG PET/CT cannot differentiate local residue from short-term active inflammation induced by SCRT and chemotherapy. In addition, [18F]FDG uptake might be confused with physiologic intestinal accumulation and activity by cytotoxic T-cell infiltration (11). In addition to intestinal inflammation, a minor degree of fibrosis might exist in the intestinal wall within 3 mo after SCRT (19), as could explain the elevated [68Ga]Ga-FAPI-04 uptake. Though FAP immunohistochemistry proved useful in correlation with PET findings, its application was impeded by the need for sequential cross-sectioning of all resected specimens to ascertain whether the patient attained pCR.
It is exciting that certain quantitative PET parameters could distinguish patients with pCR and that the [68Ga]Ga-FAPI-04 PET parameters performed better than the [18F]FDG PET parameters. Joye et al. reviewed 25 published studies on [18F]FDG PET/CT for pCR prediction after neoadjuvant therapy in rectal cancer and concluded that it has insufficient accuracy for predicting pCR (20). Recently, Backhaus et al. and Chen et al. analyzed the value of [68Ga]Ga-FAPI PET in assessing the pathologic response to neoadjuvant chemotherapy in breast cancer (21,22). Their studies revealed that [68Ga]Ga-FAPI PET/MRI had impressive performance for predicting pCR. Active cancer-associated fibroblasts in the stroma are strongly associated with reduced tumor-infiltrating immune cells and function. [68Ga]Ga-FAPI PET has unique advantages in reflecting cancer-associated fibroblast abundance and stromal reaction, making it more suitable for monitoring the response to immunotherapy than is [18F]FDG PET (14). In particular, [68Ga]Ga-FAPI-04 ΔSULpeak% exhibited high efficacy in predicting pCR. SULpeak demonstrated superior noise resilience and repeatability over SUVmax, particularly in small lesions, rendering it preferable for evaluating therapy response over the commonly used SUVmax (23). It is expected that when [68Ga]Ga-FAPI-04 ΔSULpeak% surpasses the predefined threshold (63.92%), patients will attain pCR, eliminating the need for surgical intervention and permitting a watch-and-wait strategy.
This study presented several additional findings. Accurate lymph node staging is essential in the management of rectal cancer. Compared with [68Ga]Ga-FAPI-04 PET, both [18F]FDG PET and MRI exhibited lower accuracy in detecting metastatic lymph nodes during preoperative evaluation, aligning with previous studies (13,24). In this study, the baseline neutrophil count and preoperative interleukin-4 level were significantly higher in the pCR group than in the non-pCR group. Similarly, Sun et al. found that a higher neutrophil count at baseline correlated with an increased rate of pCR (25). Meanwhile, previous studies found that elevated interleukin-4 levels were associated with a better prognosis, which plays a crucial role in the immune cells’ survival and improvement (26,27).
This study had several limitations. First, the number of investigated patients was limited, as was the follow-up time. Second, more suitable preoperative evaluation time points are worth exploring, given the existence of acute tissue inflammation and chronic fibrosis after treatment. Therefore, the research findings require additional validation by including more meticulously selected prospective cohorts.
CONCLUSION
Our study revealed the clinical significance of [68Ga]Ga-FAPI-04 PET/MRI as a promising imaging modality for predicting pCR after SCRT combined with immunochemotherapy. The PET parameter [68Ga]Ga-FAPI-04 ΔSULpeak% exhibited remarkable specificity in distinguishing patients with pCR and may further provide valuable guidance in selecting patients who can forgo surgery after neoadjuvant therapy.
DISCLOSURE
This work was financially supported by the National Natural Science Foundation of China (82030052, 81901783, and 82373050), the CSCO-Xinda Oncology Immunotherapy Research Fund (Y-XD202002-0168), and the Medical Seed Research Fund for Colorectal Cancer and Head and Neck Cancer (ZLIC-2022). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Neoadjuvant therapy in patients with LARC has achieved good pCR rates. However, there are no effective methods for predicting pCR under new immunochemotherapy regimens.
PERTINENT FINDINGS: In this prospective study involving LARC patients, [68Ga]Ga-FAPI-04 PET/MRI, [18F]FDG PET/CT, and contrast-enhanced MRI were performed on each patient before treatment initiation and after receipt of SCRT plus immunochemotherapy. The [68Ga]Ga-FAPI-04 PET parameter ΔSULpeak% had the largest area under the curve, with a high specificity of 100.0% to predict pCR.
IMPLICATIONS FOR PATIENT CARE: [68Ga]Ga-FAPI-04 PET/MRI is promising for predicting pCR after neoadjuvant therapy, and ΔSULpeak% may guide the selection of patients who can forgo surgery.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 7, 2024.
- Accepted for publication August 13, 2024.