Abstract
Rheumatoid arthritis is an autoimmune disease resulting in chronic synovial inflammation. Molecular imaging could be used to monitor therapy response, thus enabling tailored therapy regimens and enhancing therapeutic outcome. Here, we hypothesized that response to etanercept could be monitored by radionuclide imaging in arthritic mice. We tested 3 different targets, namely fibroblast activation protein (FAP), macrophages, and integrin αvβ3. Methods: Male DBA/1J mice with collagen-induced arthritis were treated with etanercept. SPECT/CT scans were acquired at 1, 24, and 48 h after injection of 111In-RGD2 (integrin αvβ3), 111In-anti-F4/80-A3-1 (antimurine macrophage antibody), or 111In-28H1 (anti-FAP antibody), respectively, with nonspecific controls included. Mice were dissected after the last scan, and scans were analyzed quantitatively and were correlated with macroscopic scoring. Results: Experimental arthritis was imaged with 111In-28H1 (anti-FAP), 111In-anti-F4/80-A3-1, and 111In-RGD2. Tracer uptake in joints correlated with arthritis score. Treatment decreased joint uptake of tracers from 23 ± 15, 8 ± 4, and 2 ± 1 percentage injected dose per gram (%ID/g) to 11 ± 11 (P < 0.001), 4 ± 4 (P < 0.001), and 1 ± 0.2 %ID/g (P < 0.01) for 111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2, respectively. Arthritis-to-blood ratios (in mice with arthritis score 2 per joint) were higher for 111In-28H1 (5.5 ± 1; excluding values > 25), 111In-anti-F4/80-A3-1 (10.4 ± 4), and 111In-RGD2 (7.2 ± 1) than for control 111In-DP47GS (0.7 ± 0.5; P = 0.002), 111In-rat IgG2b (0.5 ± 0.2; P = 0.002), or coinjection of excess RGD2 (3.5), indicating specific uptake of all tracers in arthritic joints. Conclusion: 111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2 can be used to specifically monitor the response to therapy in experimental arthritis at the molecular level. Further studies, however, still need to be performed.
Rheumatoid arthritis is an autoimmune disease with a global prevalence of 0.24% in 2010 (1). Arthritis is marked by symptoms including joint pain and swelling caused by chronic inflammation in synovial joints and can be treated systemically or intraarticularly by either nonsteroidal antiinflammatory drugs, corticosteroids, or disease-modifying antirheumatic drugs (DMARDs) (2,3). In addition, treatment with biologicals has been shown to be quite effective in patients.
Because arthritis is a chronic disease characterized by disease exacerbations (4), it has become crucial to develop tools to effectively monitor disease progression, prevent progressive destruction, and predict or monitor response to therapy, which are as important for a positive outcome as early diagnosis. Monitoring response to therapy could allow for the therapeutic schedules—aimed to reduce inflammation, relieve pain, and reduce disabilities—to be altered if deemed necessary. This could thus minimize unwarranted side effects and irreversible joint damage. This is especially pertinent for therapies such as DMARDs, in which it can take several weeks to months for clinical improvements to show (5).
Many cell types that play a role in joint inflammation and destruction are present in the synovial lining. One of the most prominent cell populations is activated fibroblastlike synoviocytes (6), which express fibroblast activation protein (FAP) (7) and could be imaged by micro-SPECT using radiolabeled antibodies against FAP, as shown by us recently (8). Other cell types include osteoclasts (9), which express integrin αvβ3 (10). Integrin αvβ3 is a much-used target for imaging tumor cells and angiogenesis in oncology using radiolabeled RGD peptides. Preclinically, F4/80 receptor–positive macrophages also play a role in arthritis pathogenesis (11). These targets have previously been successfully imaged by either radiolabeled RGD-based peptides (12) or an anti-F4/80-A3-1 labeled with 111In (13), albeit in an oncologic setting.
Noninvasive imaging could be a great tool in determining early on whether therapies are successful (14,15), yet few approaches have been aimed at a molecular level specific for arthritis. Here, we focused on imaging these 3 different targets, namely FAP, macrophages, and integrin αvβ3, and determined whether they could be useful tools to monitor response to the tumor necrosis factor (TNF) receptor fusion protein etanercept therapy in arthritic mice. These targets were also chosen because the treatment used here, etanercept, targets TNF, which is produced mainly by monocyte-macrophages, activated endothelial cells, which also express integrin αvβ3 (16), and synovial fibroblasts (17).
MATERIALS AND METHODS
Antibody Conjugation and RGD Peptide
Rat antimouse anti-F4/80-A3-1 antibody (AbD Serotec) and rat IgG2b (R&D Systems) were dialyzed against 1:1 phosphate-buffered saline (pH 7.4):water to remove sodium azide. Antibodies 28H1 (anti-FAP), DP47GS (8), anti-F4/80-A3-1, and rat IgG2b (13) were conjugated with isothiocyanatobenzyl–diethylenetriamenepentaacetic acid (p-SCN-Bz-DTPA) (Macrocyclics, Inc.) in 0.1 M NaHCO3, pH 9.5, using a 5-fold (28H1, DP47GS) or 10-fold (anti-F4/80-A3-1, IgG2b) molar excess of p-SCN-Bz-DTPA for 1 h at room temperature. Unconjugated p-SCN-Bz-DTPA was removed by dialysis against 0.25 M ammonium acetate buffer, pH 5.5 (28H1, DP47GS), or 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES), pH 5.5 (anti-F4/80-A3-1, IgG2b). DP47GS and rat IgG2b antibodies served as isotype controls for 28H1 and anti-F4/80-A3-1, respectively.
DOTA-E-[c(RGDfK)]2 (RGD2) was acquired from Peptides International. Here we used the 28H1 and DP47GS antibodies, which are noninternalizing antibodies bearing a mutation in the Fc effector part, preventing binding to the Fc receptor. All buffers used were metal-free.
The affinity of all 3 tracers and the immunoreactive fraction of anti-F4/80-A3-1 (75%) have been determined previously (8,13,18). The immunoreactive fraction for 111In-28H1 was 91.4% (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org).
Radiolabeling
For radiolabeling, 750 μg of DTPA-28H1 or DTPA-DP47GS were incubated with 250–320 MBq of 111InCl3 (Mallinckrodt BV) in 0.1 MES buffer (pH 5.4) for 30 min at room temperature. For studies involving anti-F4/80-A3-1 or rat IgG2b, 280–320 MBq of 111InCl3 (Mallinckrodt) were added to 140 μg of DTPA-anti-F4/80-A3-1 or 125 μg of rat IgG2b for 1 h at room temperature in 0.1 MES buffer (pH 5.4). For studies involving RGD2, 8 μg of DOTA-conjugated peptide, dissolved in 0.1 M MES buffer (pH 5.4), were incubated with 200 MBq of 111InCl3 for 20 min at 95 °C. Unincorporated 111In was complexed by adding ethylenediaminetetraacetic acid (final concentration of 5 mM) after the labeling.
Labeling efficiencies of all antibodies and the RGD2 peptide were determined by instant thin-layer chromatography on instant thin-layer chromatography silica gel strips (Agilent Technologies), with 0.1 M acetate buffer, pH 5.4, as the mobile phase. Labeling efficiencies were 93%, 99%, 91%, 95%, and 99% for 111In-28H1, 111In-DP47GS, 111In-anti-F4/80-A3-1, 111In-rat IgG2b, and 111In-RGD2, respectively. Tracers were diluted with phosphate-buffered saline (with 0.5% bovine serum albumin), and unlabeled antibody or peptide was added to obtain the required antibody or peptide dose.
Animal Model
Mice (22–25 g) were housed in individually ventilated cages, with 5 mice per cage in a temperature- and humidity-controlled room with a 12/12-h light/dark cycle. Animals had unlimited access to water and food. Arthritis was induced in male DBA/1J mice by intradermal immunization at the tail base with 100 μg of bovine type II collagen (CII) in Freund’s complete adjuvant, followed by an intraperitoneal booster injection of 100 μg of CII in phosphate-buffered saline 3 wk later (collagen-induced arthritis [CIA]). Arthritis scores per paw ranged from mild to severe (score: 0, no sign of arthritis; 0.25, 1–2 toes red or swollen; 0.5, 3–5 toes red or swollen; 1, swollen ankle; 1.5, swollen footpad; 2, severe swelling and ankylosis). Etanercept treatment (10 mg/kg, 3× per week, intraperitoneally) started when the mice showed the first signs of the onset of arthritis (score ≥ 0.25 in 1 joint). Untreated arthritic mice were used as a control. Animal experiments were approved by the local animal welfare committee and performed according to national regulations.
Biodistribution Studies
In dose-finding studies, arthritic male DBA/1J mice were injected intravenously with either 10 or 40 μg of 111In-anti-F4/80-A3-1 or 111In-IgG2b, as done previously for 111In-28H1, and the biodistribution was measured by ex vivo analysis of the accumulation of the tracer in different organs and joints after dissection of the mice. In other biodistribution studies, treated and untreated male mice were injected intravenously with 50 μg of 111In-DTPA-28H1 or 111In-DTPA-DP47GS, 10 μg of 111In-anti-F4/80-A3-1 or 111In-rat IgG2b, or 1 μg (radiolabeled) or 50 μg (1 μg + 49 μg unlabeled) of 111In-RGD2 (3–4 MBq). At 48 h (111In-DTPA-28H1 and 111In-DTPA-DP47GS), 24 h (111In-anti-F4/80-A3-1 and 111In-rat IgG2b), and 1 h (111In-RGD2) after tracer injection, mice were euthanized by CO2/O2 asphyxiation. Ten mice were used per group for which blood, arthritic joints, and major organs and tissues were dissected, weighed, and counted in a shielded well-type γ-counter (Perkin-Elmer) to provide the percentage injected dose per gram (%ID/g).
Micro-SPECT/CT Imaging
In imaging studies, treated and untreated male mice were injected intravenously with 50 μg of 111In-DTPA-28H1 or 111In-DTPA-DP47GS, 10 μg of 111In-anti-F4/80-A3-1 or 111In-rat IgG2b, or 1 μg (radiolabeled) or 50 μg (1 μg + 49 μg unlabeled) of 111In-RGD2 (15–21 MBq). At 48 h (111In-DTPA-28H1 and 111In-DTPA-DP47GS), 24 h (111In-anti-F4/80-A3-1 and 111In-rat IgG2b), and 1 h (111In-RGD2) after tracer injection, mice were euthanized by CO2/O2 asphyxiation for ex vivo biodistribution studies (3 mice/group). Mice were then scanned with the U-SPECT-II/CT scanner (MILabs) while in the prone position. Micro-SPECT scans were acquired as 3 frames of 15 min using a 1.0-mm pinhole ultrahigh-sensitivity mouse collimator, followed by a CT scan for anatomic reference (65 kV, 615 μA). Scans were reconstructed and all frames combined with software from MILabs, using an ordered-subset expectation maximization algorithm, with a voxel size of 0.4 mm. For high-resolution micro-SPECT imaging of an arthritic joint, a focal image was acquired using a 0.35-mm ultrahigh-resolution pinhole collimator at 9 frames of 18 min. Images were reconstructed to a voxel size of 0.125 mm without attenuation correction.
Quantitative SPECT Analysis
Reconstructed micro-SPECT scans were coregistered with CT images using Inveon Research Workplace software (version 3.0; Siemens Preclinical Solutions, LLC). The regions of interest were drawn using CT images and transferred to the SPECT data to obtain mean voxel intensities of the SPECT data corresponding to those regions of interest. Mean voxel intensity values were converted to %ID using decay correction and a standard curve (mean voxel intensity vs. kBq) acquired by scanning and reconstructing known 111In activities from 15 to 310 kBq under the same conditions as the animal scans.
Statistical Analysis
Statistical analysis was performed using the Wilcoxon signed-rank test or 2-way ANOVA with GraphPad Prism (version 5.03; GraphPad Software). Each paw was analyzed as an independent event. Spearman r correlation values between arthritis-to-blood values and arthritis score were calculated for Figure 1. Statistical significance was represented as P ≤ 0.05), P ≤ 0.01, and P ≤ 0.001.
Joint uptake of tracers in untreated or etanercept-treated CIA mice at 48 h (111In-28H1, 111In-DP47GS; A and B), 24 h (111In-anti-F4/80, 111In-ratIgG2b; C and D), and 1 h after injection (111In-RGD2 with/without excess unlabeled; E and F). Data are average ± SD (n = 7–10 mice/group [A–D] or 2 mice/group [E and F], 4 joints per mouse [A, C, and E]) or individual joints (n = 14–20 mice/group, 4 joints per mouse; B, D, and F). *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001. RA = rheumatoid arthritis.
RESULTS
Dosing Study of 111In-Anti-F4/80-A3-1
The optimal dose of 111In-anti-F4/80-A3-1 in mice was determined to be 10 μg; accumulation in most tissues remained largely unaltered compared with 40 μg of 111In-anti-F4/80-A3-1, yet the arthritis-to-blood ratio was increased (Supplemental Fig. 2). For instance, uptake of 10 or 40 μg of 111In-anti-F4/80-A3-1 in the blood equaled 1.4 ± 0.4 or 2.3 ± 0.6 %ID/g, respectively. Notably, splenic uptake was higher at 10 μg of 111In-anti-F4/80-A3-1 (103.9 ± 6.4 %ID/g) than at 40 μg of 111In-anti-F4/80-A3-1 (34.6 ± 4.4 %ID/g). The arthritis-to-blood ratios were higher for 10 μg of 111In-anti-F4/80-A3-1 (10.1 ± 5.2) than for 40 μg of 111In-anti-F4/80-A3-1 (4.6 ± 3.2), and both values were higher than for 111In-IgG2b, which equaled 0.3 ± 0.2 and 0.4 ± 0.3 at 10 and 40 μg, respectively (Supplemental Fig. 2C).
Etanercept Therapy
Treatment with etanercept was successful because macroscopic scores of arthritis were lower in treated mice than in untreated mice (Supplemental Fig. 3). At day 6 after the start of therapy, average arthritis scores were 4.0 ± 1.7 in untreated mice and 1.8 ± 1.1 in treated mice, as measured on a scale of 0–8 per mouse (i.e., 0–2 per paw).
Biodistribution Studies
Biodistribution studies after dissection showed that uptake of 111In-28H1 (Spearman r, 0.4069; P < 0.001), 111In-anti-F4/80-A3-1 (Spearman r, 0.7542; P < 0.0001), and 111In-RGD2 (Spearman r, 0.6527; P < 0.01) was decreased in arthritic joints in mice treated with etanercept and that tracer uptake correlated with arthritis score (Fig. 1). For instance, joint uptake of 111In-28H1 in treated and untreated mice averaged 11 ± 11 %ID/g (95% confidence interval [CI], 7.0–15.1) and 23 ± 15 %ID/g (95% CI, 17.6–29.1), respectively (P < 0.001). Joint uptake of 111In-anti-F4/80-A3-1 decreased from 8 ± 4 %ID/g (95% CI, 6.8–9.3) to 4 ± 4 %ID/g (95% CI, 2.5–4.9) after treatment (P < 0.001 for both tracers between treated and untreated) as did 111In-RGD2 uptake (from 2 ± 1 %ID/g [95% CI, 1.5–2.8] to 1 ± 0.2 %ID/g [95% CI, 0.8–1.2]) (P = 0.0078). Uptake of all 3 tracers when normalized to blood (arthritis-to-blood values) was higher at each arthritis score (0.25–2) for 111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2 than for control 111In-DP47GS, 111In-antiratIgG2b, and 111In-RGD2 plus unlabeled excess (Figs. 1B, 1D, and 1F). P values at arthritis score 2 (per joint) were 0.002, comparing 111In-28H1 and 111In-anti-F4/80-A3-1 with their controls.
In untreated mice, blood levels for 111In-DP47GS were higher, whereas femur uptake values were lower, than for 111In-28H1 (Supplemental Fig. 4A). Etanercept therapy resulted in lower blood values and higher liver values for 111In-28H1 and 111In-DP47GS than untreated mice (Supplemental Fig. 4A), most likely due to a mouse–antihuman IgG response. Whole-body biodistribution varied between 111In-anti-F4/80-A3-1 and its isotype-matched control 111In-ratIgG2b, with lower blood circulating levels and higher uptake in the spleen, liver, and femur than blood values for 111In-anti-F4/80-A3-1 (Supplemental Fig. 4B). Etanercept treatment had no effect on general biodistribution of 111In-anti-F4/80-A3-1, 111In-ratIgG2b, 111In-RGD2, or 111In-RGD2 plus excess unlabeled (Supplemental Figs. 4B and 4C).
Table 1 shows a summary of the arthritis-to-blood values obtained in biodistribution studies.
Mean and 95% CIs of Arthritis-to-Blood Uptake Ratios from Figures 1B, 1D, and 1F
Quantitative SPECT Analysis
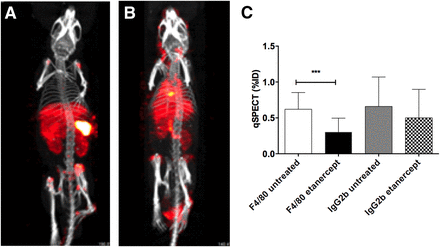
Arthritic joints were specifically visualized with 111In-28H1 (Fig. 2; Supplemental Fig. 5), 111In-anti-F4/80-A3-1 (Fig. 3; Supplemental Fig. 6), and 111In-RGD2 (Fig. 4; Supplemental Fig. 7). Quantitative SPECT analysis showed that tracer uptake within joints significantly decreased in treated animals compared with untreated controls (Figs. 2–4). For instance, joint uptake equaled 2.0 ± 0.6, 0.6 ± 0.2, and 0.2 ± 0.1 %ID in untreated animals for 111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2, respectively. These values were higher than in treated animals (0.7 ± 0.8, 0.3 ± 0.2, and 0.08 ± 0.02 %ID). Uptake of 111In-28H1 in arthritic, untreated joints was far higher (2.0 ± 0.6 %ID) than uptake of 111In-DP47GS (0.8 ± 0.4 %ID), and joint uptake of 111In-antiratIgG2b was not significantly decreased in untreated versus treated mice (0.7 ± 0.4 and 0.5 ± 0.4 %ID, respectively).
Three-dimensional SPECT/CT scans of etanercept-treated CIA mice 48 h after injection of 111In-28H1 (A) and 111In-DP47GS (B). All images are scaled equally. (C) Quantitative analysis of SPECT images. Data are average ± SD (n = 3 mice/group, 4 joints/mouse). **P ≤ 0.01.
Three-dimensional SPECT/CT scans of etanercept-treated CIA mice 24 h after injection of 111In-anti-F4/80 (A) and 111In-ratIgG2b (B). All images are scaled equally. (C) Quantitative analysis of SPECT images. Data are average ± SD (n = 3 mice/group, 4 joints/mouse). ***P ≤ 0.001.
Three-dimensional SPECT/CT scans of untreated CIA mice 1 h after injection of 111In-RGD2 (A). (B) Quantitative analysis of SPECT images of joint uptake after injection of 111In-RGD2 in untreated or treated CIA mice. Data are average ± SD (n = 1–2 mice/group, 4 joints/mouse).
Values obtained for 111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2 by quantitative SPECT analysis correlated well with macroscopic arthritis scores (Supplemental Fig. 8).
DISCUSSION
The data acquired in the dosing study were consistent with the idea of the spleen and liver acting as an antigen sink for 111In-anti-F4/80-A3-1 due to the presence of a large number of macrophages (13). Because of its short circulation time, studies involving 111In-anti-F4/80-A3-1 were completed at 24 h after injection.
Etanercept, the drug of choice in these studies, is a soluble fusion protein consisting of human p75 TNF-α receptor and the Fc portion of human IgG (19). It is a biologic DMARD that inhibits binding of inflammatory cytokine TNF-α to its receptor and is often given to arthritis patients if other treatments are contraindicated or withdrawn because of adverse events.
Here, we showed that the effect of etanercept on macroscopic/clinical arthritis score can not only be visualized noninvasively using imaging tracers that target either FAP, macrophages, or integrins, but also can be measured quantitatively in either ex vivo biodistribution studies or quantitative SPECT. In previous studies, the authors did not see accumulation of any of the tracers in the joints of nonarthritic mice (8,12,13,18). The 3 tracers are different with regard to their targets, mechanism, and pharmacokinetics; they all measure something different, so a straight comparison was not performed here.
The uptake of 111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2 is antigen/receptor-mediated because joint-to-blood values decreased for antibody controls or for 111In-RGD2 when competed with excess peptide (Fig. 1). For all tracers, including the RGD peptide, the enhanced permeability and retention effect did add to total tracer uptake because the nonspecific uptake of the tracer, displayed by total joint uptake values of 111In-DP47GS, 111In-ratIgG2b, and 111In-RGD2 plus excess, was altered in treated mice (Fig. 1).
Decreased antigen-mediated uptake of 111In-28H1 in arthritic joints of treated CIA mice could be explained by diminished expression of FAP at the site, which fits the notion of etanercept inducing apoptosis in activated fibroblastlike synoviocytes (20). The potential presence of antidrug antibodies, as seen in arthritis cases for anti-TNF drugs, such as etanercept (21,22), human antimurine antibodies (23), or antihuman murine antibodies (24), could explain the enhanced liver uptake of 111In-28H1 after etanercept treatment (Supplemental Fig. 4A). In turn, this enhanced liver uptake might also explain the lower uptake in joints of treated mice.
Decreased uptake of 111In-anti-F4/80-A3-1 in arthritic joints after treatment may also be explained by lowered targeted availability because etanercept has been shown to induce apoptosis in macrophages resident in the synovium of arthritis patients at 8 wk as well as in mononuclear cells from synovial fluid in vitro (25). Similar targeted-mediated responses are also thought to be responsible for changes in 111In-RGD2 uptake in arthritic joints after etanercept therapy by affected osteoclasts (26,27) and vasculature (28). The latter option is possible, because endothelial cells have previously been targeted for use in imaging of inflammation (29).
Quantitative SPECT values (%ID) and biodistribution data (%ID/g) were not directly comparable in these studies, because quantitative SPECT values were derived from the whole paw (toe/finger to above ankle/wrist) and biodistribution data were derived from the areas directly surrounding the ankle/wrist. Interestingly, total non–antigen-mediated uptake of 111In-ratIgG2b in the joint significantly decreased in treated compared with untreated CIA mice in biodistribution studies (Fig. 1C) yet not in the quantitative SPECT studies (Fig. 3C), likely because of a limited sensitivity of quantitative SPECT.
Because of the large variation in arthritis scores induced in the CIA mice and different responses to etanercept in individual mice, many animals had to be used per group. Despite this, the correlative figures show that these tracers can be used to objectively and quantitatively determine arthritis score in individual paws.
Put et al. provide a review on general tracers and radiolabeled biologicals used to image inflammatory processes (30). Previous studies have focused mostly on targeting macrophages—either with radiolabeled folate (31–33), with (R)-11C-PK11195 or translocator protein TSPO (34–36) (which has also been used clinically), or with radiolabeled nanobodies (37)—or by targeting CD163 receptors (38) or mannose receptors (39). Unfortunately, images acquired using radiolabeled folate led to poor-quality images (31), most probably due to general low uptake of the tracers in the joints (32), a feature also seen with the anti-CRIg Nanobody 99mTc-NbV4m119 (37). So far, the only tool to image F4/80 receptors specifically has been near-infrared-labeled antibodies (40), making our radiolabeled anti-F4/80-A3-1 antibody a unique nuclear tool.
Other, more generalized ways of imaging arthritis include 3′-deoxy-3′-18F-fluorothymidine (41), which showed high background levels due to uptake in bone marrow, and 18F-FDG. Despite the fact that 18F-FDG was suggested as a good tool to develop the therapeutic effect of novel therapies of inflammatory arthritis in the study by Kundu-Raychaudhuri et al. (42), in a preclinical model Laverman et al. showed that 18F-FDG was inferior to other nuclear imaging tracers when correlating uptake of 18F-FDG with arthritis score (8).
Monitoring response to therapy in arthritis is usually performed by clinical macroscopic scoring, ultrasonography, conventional radiography, or MRI. This highlights not only the originality of the studies performed here, by targeting integrins, macrophages, or FAP in preclinical models of arthritis, but also the novelty of assessing these tracers as tools to monitor therapy response. Radionuclide-imaging-targeting-specific molecular mechanisms could therefore be a great tool alongside the standard techniques, although cost–benefit analysis will need to be performed to determine whether any potential risk from radiation dose outweighs the benefits of early and quick therapy response monitoring.
CONCLUSION
111In-28H1, 111In-anti-F4/80-A3-1, and 111In-RGD2 can be used to specifically monitor therapy response in experimental arthritis at the molecular level. Further studies, however, still need to be performed not only to confirm that altered target expression is the cause of the diminished uptake of all 3 tracers, but also to determine whether these tracers could be used for early diagnosis of arthritis as well as whether they can be used to monitor response to other types of therapies.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. A portion of this study was funded by the Roche Postdoc Fellowship (RPF) Program. Tapan K. Nayak, Anne Freimoser-Grundschober, and Christian Klein are all employed by Roche, who provided the 28H1 and DP47GS antibodies. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Tessa van der Geest, Danny Gerrits, and Janneke Molkenboer-Kuenen for help with the biodistribution studies.
Footnotes
Published online Dec. 3, 2015.
- © 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.