Abstract
The α-emitter 211At labeled to a monoclonal antibody has proven safe and effective in treating microscopic ovarian cancer in the abdominal cavity of mice. Women in complete clinical remission after second-line chemotherapy for recurrent ovarian carcinoma were enrolled in a phase I study. The aim was to determine the pharmacokinetics for assessing absorbed dose to normal tissues and investigating toxicity. Methods: Nine patients underwent laparoscopy 2–5 d before the therapy; a peritoneal catheter was inserted, and the abdominal cavity was inspected to exclude the presence of macroscopic tumor growth or major adhesions. 211At was labeled to MX35 F(ab′)2 using the reagent N-succinimidyl-3-(trimethylstannyl)-benzoate. Patients were infused with 211At-MX35 F(ab′)2 (22.4–101 MBq/L) in dialysis solution via the peritoneal catheter. γ-camera scans were acquired on 3–5 occasions after infusion, and a SPECT scan was acquired at 6 h. Samples of blood, urine, and peritoneal fluid were collected at 1–48 h. Hematology and renal and thyroid function were followed for a median of 23 mo. Results: Pharmacokinetics and dosimetric results were related to the initial activity concentration (IC) of the infused solution. The decay-corrected activity concentration decreased with time in the peritoneal fluid to 50% IC at 24 h, increased in serum to 6% IC at 45 h, and increased in the thyroid to 127% ± 63% IC at 20 h without blocking and less than 20% IC with blocking. No other organ uptakes could be detected. The cumulative urinary excretion was 40 kBq/(MBq/L) at 24 h. The estimated absorbed dose to the peritoneum was 15.6 ± 1.0 mGy/(MBq/L), to red bone marrow it was 0.14 ± 0.04 mGy/(MBq/L), to the urinary bladder wall it was 0.77 ± 0.19 mGy/(MBq/L), to the unblocked thyroid it was 24.7 ± 11.1 mGy/(MBq/L), and to the blocked thyroid it was 1.4 ± 1.6 mGy/(MBq/L) (mean ± SD). No adverse effects were observed either subjectively or in laboratory parameters. Conclusion: This study indicates that by intraperitoneal administration of 211At-MX35 F(ab′)2 it is possible to achieve therapeutic absorbed doses in microscopic tumor clusters without significant toxicity.
The lifetime risk of ovarian cancer is 1%−2% in European and U.S. women. Despite seemingly successful cytoreductive surgery, followed by systemic chemotherapy, most patients will relapse and succumb. The relapse is most frequently localized in the abdominal cavity. New systemic chemotherapy regimens have not improved the outcome over the past decade, which prompted experimental intraperitoneal treatments, including radioimmunotherapy.
Radioimmunotherapy with β-emitters has displayed promising results, although an international randomized phase III study of 90Y-HMFG1 showed no improvement in survival or time to relapse (1). This disappointing result could be partly explained by the choice of radionuclide. The long range of this β-emitter results in poor irradiation of small tumor clusters, likely insufficient to eradicate peritoneal micrometastases. Furthermore, the relatively long half-life (T1/2) of 64 h of 90Y is not optimal, considering bone-marrow irradiation.
In treating micrometastases, α-emitters offer significant advantages over β-emitters. Because of the short range and high linear energy transfer of α-particles, targeted small cell clusters are more effectively irradiated. As the range of the emitted particles conforms to the size of the target cluster, a high fraction of emitted energy will be absorbed in the target. The therapeutic potential of the α-emitter 211At (T1/2 = 7.21 h) labeled to MOv18, MX35, and trastuzumab has been demonstrated in studies using a preclinical mouse ovarian cancer model (2–9).
Immunohistochemistry indicates that the antibody MX35 displays homogeneous reactivity with approximately 90% of human ovarian epithelial cancers and with a limited number of normal tissues (10). Specific tumor localization of 125I- and 131I-labeled MX35 and MX35 F(ab′)2 has been demonstrated in patients with intraperitoneal growth of ovarian cancer (11,12).
The encouraging results of using 211At-MX35 F(ab′)2 in the nude mouse model justified the present clinical phase I study. In addition to defining the pharmacokinetics of 211At-labeled MX35 F(ab′)2 to enable absorbed dose estimates, the aim was to investigate the feasibility and safety. Women relapsing after complete clinical remission after surgery and chemotherapy and reaching another remission on salvage chemotherapy were included in the study.
MATERIALS AND METHODS
Antibody and Cell Line
A clinical-grade F(ab′)2 fragment of the murine IgG1 monoclonal antibody MX35 was obtained from Memorial Sloan-Kettering Cancer Center. The antigen recognized by MX35 has recently been characterized as the sodium-dependent phosphate transport protein 2b (NaPi2b), which is overexpressed on more than 90% of human ovarian epithelial cancers (13). The human ovarian cancer cell line NIH:OVCAR-3, obtained from American Type Culture Collection, was used for immunologic in vitro control of the radiolabeled MX35 F(ab′)2 product.
211At Production and Distillation
211At was produced and distilled as previously described (14). In short, 211At was produced by means of the 209Bi(α,2n)211At reaction using a cyclotron (MC32; Scanditronix) at the PET and Cyclotron Unit, Rigshospitalet. Irradiation was performed at an internal water-cooled probe, using beam energies of 28–29 MeV and beam currents of 16.8 ± 1.5 μA. Activities of 2.0 ± 0.2 GBq, which correspond to a saturation yield of 230 ± 20 MBq/μA, were achieved with irradiation times of 7.6 ± 0.2 h. The target was transported to Gothenburg, Sweden, by car within 4 h, and then transformed into a chemically useful form by dry distillation. All glassware was autoclaved before distillation. After distillation, the 211At was eluted with 300 μL of chloroform into a 1.3-mL reaction vial. The 211At was then divided into six to eight 150-MBq fractions in 1.3-mL reaction vials, and the chloroform was evaporated under a gentle stream of nitrogen.
Labeling of MX35 F(ab′)2
The antibody fragment was labeled in clinically approved facilities at the Nuclear Medicine Department, Sahlgrenska University Hospital, using the reagent N-succinimidyl-3-(trimethylstannyl)-benzoate as previously described (15). All glassware and utensils were sterilized. Buffer solutions and chemicals were prepared at the local pharmacy. A total of 150 μg of MX35 F(ab′)2 were added to each 150-MBq fraction. The 211At-MX35 F(ab′)2 product was transferred through a 0.2-μm sterile filter into a peritoneal dialysis fluid (Extraneal; Baxter) bag. Overall radiochemical yields were in the range of 20%−30%.
Quality Control
A 50-μL aliquot of the astatinated product was taken for quality control. The radiochemical purity was determined by both methanol precipitation and fast-protein liquid chromatography (FPLC) on a Superdex-200 column using an ÄKTA-FPLC delivery system (GE Healthcare Bio-Sciences A). Only products with a radiochemical purity of greater than 95% were approved for clinical infusion.
The immunoreactive fraction of MX35 F(ab′)2 after astatination was determined by binding to NIH:OVCAR-3 cells using the method described by Lindmo et al. (16). Simultaneously with the complete assay, a single-point assay was started. To 4 tubes containing NIH:OVCAR-3 cells at a concentration of 5 × 106 cells/mL, 10 ng of the astatinated product were added. The tubes were incubated for 45 min, with a set limit of 50% immunoreactive fraction for infusion.
Patients
Nine women (age, 38–69 y; median age, 52 y) were included in the study; these women were initially successfully treated for ovarian carcinoma but later relapsed and were treated long-term with salvage chemotherapy, including paraplatin and paclitaxel, resulting in clinically and biochemically complete remission. According to the protocol, these patients were chosen for treatment at levels of increasing radioactivity. The patients were included in the study after providing informed consent according to the Ethics Committee of the Sahlgrenska University Hospital in Gothenburg.
Clinical Procedure
The treatment procedures are shown in Figure 1. Baseline hematology, liver function, creatinine levels, and human antimouse antibody (HAMA) were analyzed, and normal values were required. During laparoscopy, in which the abdominal cavity was inspected and biopsies were performed for microscopic lesions and adhesions, a Tenckhoff peritoneal catheter (Tyco Healthcare) was inserted 2–5 d before treatment. To ascertain free fluid access to the abdominal cavity, on the day before treatment, 1–2 L of 99mTc-LyoMAA (Mallinckrodt Medical) in Extraneal were infused; peritoneal scintigraphy was performed 1 h later, after which the fluid was evacuated. After the fifth patient was treated, the protocol was amended, and the ensuing patients were given potassium perchlorate (custom-made tablets, 200 mg)—2 tablets the evening before and 2 the morning of the day of treatment—to block uptake of astatine to the thyroid. The last patient (patient 9) was instead given potassium iodide (Recip tablets, 65 mg)—1 tablet the evening before and 2 on the day of treatment.
Schematic overview of logistics of therapeutic procedures. IP = intraperitoneal.
211At-MX35 F(ab′)2 (22.4–101 MBq/L) in Extraneal (1–2 L, 37°C) was infused via the peritoneal catheter over 30 min, together with 0.2 MBq of 125I-human serum albumin (HSA), a reference for in vivo stability. Treatment specifications including administered activity concentration and volume are shown in Table 1. Twenty-four hours after infusion, the remaining peritoneal fluid was drained and collected from the catheter, which was then removed. Over the first 8 h after infusion, blood and intraperitoneal fluid were sampled hourly. After this period, samples were drawn every 6 h until 48 h after infusion for blood and until drainage (24 h) for intraperitoneal fluid. Urine was collected at each voiding until 48 h. Scintigraphy was performed 1 h after infusion and every 6–12 h, totaling 3–5 scans. In addition, 6 h after infusion, a SPECT or SPECT/CT study of the thoracic and abdominal area was conducted.
Treatment Specifications and Absorbed Doses to Organs at Risk
The patients were released from the hospital 48 h after infusion and were followed weekly for the next 8 wk at the outpatient department. Follow-up consisted of the following tests: hematology together with biochemistry, including liver function, creatinine, thyroid function, CA-125, and HAMA (i.e., the same tests performed initially). Thereafter, the patients were followed according to the clinical routines.
Fluid Sample Analysis
Blood was taken in 2 separate tubes at each time point for serum and whole-blood analysis. The serum was separated by centrifugation at 500g for 10 min. From each blood, serum, and intraperitoneal fluid sample, a 1-mL aliquot was weighed and measured for the determination of 211At and 125I content. In a few patients, FPLC or methanol precipitation analyses were performed on serum or intraperitoneal fluid samples, to estimate the protein-bound fraction of 211At. Reliable urine data were obtained from only 4 patients. All urine was weighed, and a small sample from each voiding was measured for 211At and 125I content.
Scintigraphy
Planar γ-camera scans were acquired using the polonium x-rays inherent to the 211At decay for imaging. A 2-detector γ-camera system (Millenium VG; GE Healthcare) with a medium-energy collimator was used. The scans covered the area from the thyroid to the ankles. The scan speed was 10 cm/min, and the energy window was 79 keV ± 10%. A calibration factor of 7.65 counts/kBq for thyroid activity uptake was obtained for planar images using a neck phantom. SPECT or SPECT/CT was conducted using the same equipment and energy window. The SPECT scan was acquired over 20 min in 360° and 120 projections.
Radioactivity Measurements
High activity (>100 kBq) was measured in an ionization chamber (CRC-15 dose calibrator; Capintec), and low activity (<10 kBq) was measured with a thallium-doped sodium iodide γ-counter (Wizard 1480; Wallac). Samples containing both 211At and 125I were measured twice, the second time after decay of 211At. Dual-energy window settings in the γ-counter enabled spillover correction. The 2 devices were cross-calibrated for both nuclides.
Dosimetry
A simple approach, in which all but the α-particle contribution were disregarded, was used for the dosimetry. Because the microdistribution of the labeled immunoconjugate in human tissue is not known in detail, a homogenous distribution of the radioactivity was assumed. The absorbed dose, Dx, for organ x was calculated using Equation 1, in which the absorbed fraction of the α-particles,  , was assumed to be 1, the mean energy released per 211At decay, Δα, is 1.09 × 10−12 J (Bq s)−1, and the cumulated activity in organ x, Ãx, was calculated from organ uptake data using Equation 2:
, was assumed to be 1, the mean energy released per 211At decay, Δα, is 1.09 × 10−12 J (Bq s)−1, and the cumulated activity in organ x, Ãx, was calculated from organ uptake data using Equation 2: Eq. 1
Eq. 1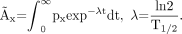 Eq. 2Function px in Equation 2 was fitted to decay-corrected uptake data for organ x. Different fit models were used for different organs. The integration was performed numerically to 48 h, corresponding to approximately 6.6 half-lives of 211At (T1/2 = 7.21 h). The absorbed doses presented in this study were not weighted for relative biologic effectiveness for 2 reasons: first, because there is no formally adopted standard for the quantity (17), and second, because reliable relative biological effectiveness values are not yet determined for all organs included.
Eq. 2Function px in Equation 2 was fitted to decay-corrected uptake data for organ x. Different fit models were used for different organs. The integration was performed numerically to 48 h, corresponding to approximately 6.6 half-lives of 211At (T1/2 = 7.21 h). The absorbed doses presented in this study were not weighted for relative biologic effectiveness for 2 reasons: first, because there is no formally adopted standard for the quantity (17), and second, because reliable relative biological effectiveness values are not yet determined for all organs included.
The bone-marrow dosimetry was based on serum activity concentration data. The activity concentration in red bone marrow is directly proportional to the serum activity concentration via the red marrow extracellular fluid fraction (18), which was estimated to be 0.19 in a study of the albumin distribution volume in the femoral bone marrow of rabbits (19). Calculated red bone–marrow concentration data were fitted to a third-degree polynomial to be used in Equation 2. For the thyroid, a second-degree polynomial was fitted to decay-corrected uptake data calculated from the planar γ-camera scans. The thyroid location was not always easily found, especially in the blocked patients. In these cases, the location was estimated; if the content was less than background, it was set to zero. A standard thyroid mass of 20 g was used for all patients. For the peritoneal lining and urinary bladder epithelium, the absorbed dose was calculated as half of the equilibrium absorbed dose to the fluid in the cavity. The intraperitoneal fluid activity concentration data were fitted to Equation 3: Eq. 3
Eq. 3
The dose calculations were made for 0–48 h, even though the data were limited to 24 h. This approach was justified by the fact that a fluid film will remain on the peritoneal surfaces after the drainage at 24 h. The urine data were entered into Equation 2 as a stepwise flat function, assuming constant activity concentration between each voiding.
RESULTS
For this treatment modality, it is the activity concentration and not the activity per se that determines the absorbed dose to tumor and to organs at risk. The absorbed doses were therefore related to the initial activity concentration, IC (MBq/L), of the infused solution. All activities were decay-corrected to the time of infusion and normalized to the starting concentration.
Fluid Sampling
The protein-bound fraction of 211At in intraperitoneal fluid was evaluated in patient 1 by FPLC analysis of samples drawn 2, 8, and 25 h after infusion. At none of the time points could significant amounts of free 211At be found. This was confirmed by methanol precipitation of intraperitoneal fluid samples from the same patient and from patient 2. The protein-bound fraction was 95% or higher. Methanol precipitation of serum was performed on samples from patients 1 and 9. The protein-bound fraction was approximately 80% in patient 1 and above 90% in patient 9 after 6 h.
A curve of the form A*exp(−Bt) + C was fitted to the intraperitoneal fluid concentration data for each patient. Extrapolation of the curve to t = 0 yielded the individual IC, to which all other data were normalized. The initial concentrations are listed in Table 1.
The concentration of both 211At-MX35 F(ab′)2 and 125I-HSA in the intraperitoneal fluid decreased continuously, reaching approximately 50% IC at 24 h. The process is shown in Figure 2 as the mean concentration measured at 1–24 h in all patients. A slight difference in concentration between the 2 proteins was observed in the early phase but later reduced. Blood and serum concentration measurements gave comparable results, the blood concentration consistently being 0.6 of the serum concentration. Therefore, blood data are not presented. The mean serum activity concentration at 1–48 h (Fig. 3) increased during treatment and continued to do so even after evacuation of the intraperitoneal fluid, reaching 6% IC for 211At-MX35 F(ab′)2 and 10% IC for 125I-HSA at 45 h. The difference between the 2 proteins was small at first but increased with time. The cumulative excretion in urine (Fig. 4) of the 2 nuclides was similar, that is, 35–40 kBq/(MBq/L) at 24 h, but at 48 h the 211At excretion was nearly twice the average 125I excretion.
Mean intraperitoneal fluid activity concentration in patients 1–9 ± SEM. Data are decay-corrected and normalized to time of infusion. hpi = hours post infusion.
Mean serum activity concentration in patients 1–9 ± SEM. Data are decay-corrected and normalized to IC in intraperitoneal fluid. hpi = hours post infusion.
Cumulative urinary activity excretion for patients 1, 3, 5, and 9. Data are decay-corrected and normalized to IC in intraperitoneal fluid. hpi = hours post infusion.
Scintigraphy
The γ-camera scans indicated that the abdominal distribution of the infused solution was good overall. For example, a series of planar anteroposterior scans of patient 5 is shown in Figure 5. The scans were obtained at 1.5, 5, 11.5, and 19.5 h after the infusion. The distribution of the therapeutic solution was similar to that of the 99mTc-LyoMAA infused on the day before the therapy. Elevated organ uptake was not found on the planar images or on the SPECT or SPECT/CT images, except in the thyroid of unblocked patients. Specifically, there was no elevated uptake in the lungs, liver, or kidneys. In addition, the infusion volume, which was varied between 1 and 2 L, did not seem to affect the distribution of the solution in the abdominal cavity.
Consecutive AP scans of abdominal and thoracic area of patient 5; thyroid uptake not blocked. Images acquired at 1.5, 5, 11.5, and 19.5 h after infusion of 211At-MX35 F(ab′)2. Gray scale is normalized to maximum pixel value of each image 82, 47, 28, and 16, respectively. AP = anteroposterior.
The thyroid uptake was evaluated from the planar γ-camera images. Individual uptake data are shown in Figure 6. The blocked patients had no or low uptake. In unblocked patients, astatine clearly accumulated in the thyroid. The variability was great, 127% ± 63% IC at 20 h after infusion, corresponding to approximately 1.4% of the administered activity. Two patients were scanned after the drainage of the intraperitoneal fluid. The data from these scans indicate that the uptake continued to increase. At 48 h, the thyroid content was 872% IC in patient 4.
Thyroid 211At activity concentration in patients 1–9 calculated from AP scans. Patient 4 had 872% IC at 48 h (cut from graph). Patients 6–8 were blocked with potassium perchlorate, patient 9 with potassium iodide. Data are decay-corrected and normalized to IC in intraperitoneal fluid. AP = anteroposterior; hpi = hours post infusion.
Dosimetry
Dosimetry was calculated individually for each patient and was related to the IC (MBq/L) of the infused solution. The absorbed dose to red bone marrow was 0.14 ± 0.04 mGy/(MBq/L), to the peritoneum it was 15.6 ± 1.0 mGy/(MBq/L), to the urinary bladder wall it was 0.77 ± 0.19 mGy/(MBq/L), to the unblocked thyroid it was 24.7 ± 11.1 mGy/(MBq/L), and to the blocked thyroid it was 1.4 ± 1.6 mGy/(MBq/L) (mean ± SD). Patient-specific absorbed doses are found in Table 1. Blocking the thyroid reduced the absorbed dose by approximately 95%.
Clinical Toxicity Outcome
No subjective toxicity related to the immunoconjugate was seen. No increase of HAMA was recorded over the 8 wk after treatment.
Hemoglobin concentration, white blood cell counts (neutrophils and lymphocytes, separately), creatinine concentration, and thyroid function were monitored for acute short-term effects for 8 wk after treatment; no changes related to serum activity concentration were found. Long-term follow-up included measurement of the above parameters until relapse, when further chemotherapy was administered, and still later. On a median follow-up of 23 mo, no hematologic, renal, or thyroid abnormality was recorded.
Only 3 patients have remained in clinical and CA-125 remission; CA-125 elevation developed in the others, and 1 has died from the disease.
DISCUSSION
Ovarian cancer is a frequent and, for most patients, deadly disease. Standard treatment is maximal cytoreductive surgery, with or without retroperitoneal lymph node dissection, followed by at least 6 courses of chemotherapy, as a rule paclitaxel in combination with carboplatin. Complete clinical remission is obtained in most patients; if a second-look laparotomy is performed after the chemotherapy is completed, however, only 1 in 3 of the patients in stage III are microscopically free of tumor, and up to 50% of those patients will have a tumor relapse. This group will probably benefit from consolidating intraperitoneal therapy.
Various β-emitters, for example 131I, 177Lu, and 90Y, have been used in intraperitoneal radioimmunotherapy (20–26). Phase I–II studies indicated considerable efficacy, but the only large-scale, well-controlled randomized phase III study using 90Y-HMFG1 unfortunately proved unsuccessful (1). Thus, the β-particles seem to be ineffective in treating minimal disease. However, analysis of subgroups in that study indicated that women with residual disease after surgery had a prolonged time to intraperitoneal relapse when exposed to radioimmunotherapy (27). In these patients, probably with larger tumors, though not visible at laparoscopy at the time of treatment, it is reasonable that the long-range β-particles would be relatively more efficient than in patients with smaller residual tumor cell clusters (i.e., less than a few hundred micrometers). Because targeted single cells or small cell clusters are considerably more effectively irradiated by α-particles (because of the short range of these α-particles), the latter patient group (i.e., those without residual disease after surgery) would probably benefit most from α-radioimmunotherapy.
In animal studies, treatment with 211At-MX35 F(ab′)2 was shown to be sterilizing for tumors less than approximately 0.5 mm in diameter, without bringing about absorbed doses that were critical to normal tissues (5). Those findings led to the phase I study of 211At-MX35 F(ab′)2, launched in 2005, including women in good remission after second-line chemotherapy for recurrent ovarian carcinoma. These patients were selected because they were not cured by conventional treatment, and they somewhat mimicked the future target patient population (i.e., up front in the primary therapy after surgery and chemotherapy). This is the second clinical study of 211At-labeled antibodies and the first one using intraperitoneal administration. In the first reported study, the 211At-labeled antibody was administered in surgically created resection cavities in the brain, resulting in insignificant systemic irradiation (28).
For most radioimmunotherapies, even for intraperitoneal administration, the bone marrow is the critical organ limiting the administered activity. In intraperitoneal administration, with infusion volumes of 1–2 L, the labeled antibodies are expected to reach the blood through the lymphatic drainage, leaving the abdominal cavity in about 2 d. When decay-corrected, the 211At-labeled antibodies displayed the same linear increase in activity concentration in blood as has been observed for 186Re-, 177Lu-, 131I-, and 90Y-labeled antibodies (20,29–31). However, compared with these radionuclides, the 211At blood content results in a much lower irradiation of the bone marrow, which is explained by the significant physical decay (T1/2 = 7.21 h) during abdominal cavity–to–blood transport. This obvious benefit of a short-lived radionuclide in localized therapy implies that the tissue affected by local irradiation, that is, the peritoneum, and not the bone marrow, might limit the administered amount of activity. Long-term studies in mice are under way using 211At- and 213Bi-conjugated antibodies to determine the absorbed dose tolerated by the peritoneum, which to our knowledge has never before been studied. In the present study, the highest absorbed dose to the peritoneal surface was moderate (1.6 Gy) and did not result in any obvious short- or long-term abdominal toxicity.
The highest absorbed dose to the bone marrow among the enrolled patients was 0.01 Gy, roughly 2.5% of the estimated tolerable absorbed dose, and was low, compared with previous systemic α-radioimmunotherapy (32). The absorbed dose to the bone marrow was calculated using a bone marrow–to–serum activity concentration ratio valid for macromolecules (0.19), because chromatography indicated that most (>80%) of the 211At in serum was bound to the antibody fragments. The ratio corresponds to a bone marrow–to–blood ratio of 0.32, a value in the range of what has been observed in previous radioimmunotherapy studies (33).
Despite a high protein-bound fraction found in serum, a small but increasing amount of free radionuclide appeared in the urine and in the thyroid. Most of this activity appeared after 24 h, when the antibodies had reached the circulation and were exposed to degradation. The degradation and subsequent release of free 211At was also indicated by the increasing difference in plasma concentrations of 211At-MX35 F(ab′)2 and 125I-HSA, the latter being the more stable conjugate, having a long half-life in blood and not being filtered in the kidneys. If not blocked, the irradiation of the thyroid is significant. After the introduction of a blocking agent, the thyroid uptake was significantly reduced, thereby reducing by approximately 95% the absorbed dose to an acceptable level. No change in thyroid-stimulating hormone levels was seen in either unblocked, low-activity patients or in blocked, higher-activity ones.
The stomach is another organ known to accumulate free 211At via the sodium/iodide symporter receptor, though neither planar nor SPECT images revealed any uptake distinguishable from background activity. However, a significant uptake in the thin outspread mucosa could have been overlooked against the high abdominal background activity. It is likely that the thyroid blocking agent may also block any potential uptake of free 211At in the stomach (34); the risk of significant radiation damage to stomach tissue was, therefore, considered low. The other major organs of interest, that is, the liver, lungs, and kidneys, did not display any uptake. The MX35 antigen has been detected in normal human tissues, including the epithelial cells of the normal bronchus, lungs, sweat glands, kidney collecting ducts, thyroid, fallopian tubes, cervix, and uterus (10). Thus, although not detected by the γ-camera, these tissues might accumulate 211At-MX35 F(ab′)2. Because the systemic irradiation in general is low, the irradiation of these tissues is probably well below the tolerable level.
The binding of the antibodies to the tumor cells in micrometastases will most likely occur via the peritoneal fluid and not via vascular flow. Thus, the activity concentration of 211At-MX35 F(ab′)2 in the peritoneal fluid determines the irradiation of the microscopic peritoneal tumors. In this study, the decay-corrected fluid concentration of radiolabeled antibody declined with time, contrary to previous studies finding a stable or increasing concentration over 24 h (29,31). This difference is likely explained by the osmotic agent used in our study, Extraneal, which results in water influx into the abdominal cavity. Extraneal was used to guarantee a well-filled abdominal cavity during the time of irradiation, that is, for 24 h.
Animal data indicate that a concentration of approximately 200 MBq/L is needed for high therapeutic efficacy (8). To achieve that concentration for our patients, we aimed at a maximum concentration of 400 MBq in 2 L. Because the availability of labeled product was restricted by limited production capacity of 211At and moderate labeling yields, the volume was reduced to 1 L during the study to enable escalation of activity concentration. Yet, only a concentration of 100 MBq/L was attained. The reduced volume does not affect tumor irradiation as long as the peritoneal surface is completely exposed throughout the irradiation time (i.e., over ∼24 h), because the binding of antibodies to tumor cells and unspecific irradiation of the peritoneum are concentration-dependent. This criterion is most likely also fulfilled with a volume of 1 L when Extraneal is used. From the γ-camera images, we could distinguish no difference in distribution between patients who received different volumes.
We have chosen to report biokinetic data and absorbed doses related to the initial peritoneal fluid activity concentration (IC). The common way to provide biokinetic data is to relate them to the administered activity, presented in the form of percentage injected dose per gram. This form is not applicable in the present study. This seemingly odd statement is explained by the short half-life of 211At in relation to the abdominal fluid–to–blood transport time for infusion volumes in the range of 1–2 L. Almost all 211At activity has decayed before the fluid in the abdominal cavity is emptied, suggesting that the total volume, and consequently the total activity, is of little importance. For a constant lymphatic flow draining the abdominal cavity of the 211At-labeled antibody, the time-dependent activity concentration in blood and various tissues depends on the activity concentration in the peritoneal fluid. Therefore, maximum tolerated dosage should be stated in terms of activity concentration.
The highest administered activity concentration in this study, 100 MBq/L, did not result in any side effects. Thus, the concentration is safe. Furthermore, the organ doses resulting from this concentration were much lower than expected tolerance levels. One exception could be the peritoneal surface, for which the margin of toxicity is yet unknown. Consequently, the maximum tolerated activity concentration cannot be stated until the radiosensitivity of the peritoneal membrane has been investigated. Increased activity of 211At-labeled antibody is now available, because labeling yield has been considerably improved (35).
It is beyond the scope of the present study to validate the therapeutic efficacy for the studied patients. Laparoscopic examination of the peritoneum before treatment did not reveal any tumor growth in any of the patients, but most likely microscopic peritoneal tumors and retroperitoneal lymph node invasion are present in this patient category. The highest administered concentration, 100 MBq/L, might be sterilizing for single cells and small cell clusters (7). However, the rate and amount of 211At-MX35 F(ab′)2 binding to the tumor cells is critically dependent on the antigen expression. Approximately 10% of ovarian cancers do not express the MX35 antigen (10). The enrolled patients were not tested for this antigen expression because the aim of the present phase I study was to clarify the pharmacokinetics of the conjugate and potential side effects, which were not assumed to be dependent on the tumor cell antigen presentation.
CONCLUSION
The pharmacokinetic information obtained here indicates that therapeutic absorbed doses to micrometastases in the abdominal cavity may be delivered by the intraperitoneal administration of 211At-MX35 F(ab′)2 without observed or estimated toxicity. However, the maximum tolerated activity concentration cannot yet be established because the tolerable absorbed dose to the peritoneum is unknown.
Acknowledgments
Ann-Christine Bergh and Irma Nikadon are acknowledged for assistance with scintigraphy, Ingela Claesson for cell culturing, Börje Haraldsson for consultation on peritoneal transport mechanisms and help with the study design, Sofia Frost for help with the protocol and quality control, and Pernilla Dahm-Kähler for laparoscopy and catheter insertion. This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, and the King Gustaf V Jubilee Clinic Research Foundation in Gothenburg, Sweden.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication January 29, 2009.
- Accepted for publication March 18, 2009.













