Abstract
High-frequency anterior capsular stimulation is a new, promising, and reversible neuromodulatory treatment in the research stage for patients with refractory obsessive-compulsive disorder (OCD). The mechanism of action is unknown but hypothesized to be secondary to interruption of the corticothalamostriatocortical circuit. Methods: 18F-FDG PET was performed on 6 consecutive OCD patients preoperatively and after stimulation. The results were compared with those of 20 age- and sex-matched healthy volunteers by using both a standardized volume-of-interest–based approach for subcortical areas and statistical parametric mapping. Correlations were investigated with Yale-Brown Obsessive Compulsive Scale scores (Y-BOCS) and Hamilton Depression Rating Scale scores (HAM-D). Results: Chronic anterior capsular electrostimulation resulted in a further decrease of prefrontal metabolic activity, especially in the subgenual anterior cingulate (P < 0.001). Correlation analysis demonstrated that decreases in Y-BOCS and HAM-D with anterior capsular electrostimulation were inversely related to the metabolic activity changes in the left ventral striatum, left amygdala, and left hippocampus (P < 0.01). Preoperative resting metabolic activity in the subgenual anterior cingulate was predictive of therapeutic response (P = 0.001). Conclusion: These observations provide evidence that the subgenual anterior cingulate and ventral striatum have a key role in the neuronal circuitry involved in the pathophysiology of OCD with associated major depression and in the neuromodulatory mechanism of anterior capsular stimulation.
Obsessive-compulsive disorder (OCD) is an anxiety disorder with a lifetime prevalence of 1%–3% and for which selective serotonin reuptake inhibitors or cognitive behavioral therapy is the treatment of choice (1). It is estimated that at least 10% of patients are treatment refractory (2). A subset of patients with severe, chronic, and highly refractory OCD has undergone irreversible neurosurgical approaches such as anterior cingulotomy, subcaudate tractotomy, limbic leukotomy, and anterior capsulotomy (3). Anterior capsular electrostimulation has been investigated as an adjustable and reversible neuromodulatory approach (4,5) and was shown to result in long-term, effective reduction of the severity of OCD and associated depressive symptoms (6).
Dysfunctional corticostriatothalamocortical circuitry has previously been implicated in OCD. The most consistent neuroimaging finding in patients with OCD symptomatology alone is a relative hypermetabolism of the orbitofrontal cortex and anterior cingulate cortex that can be reduced by pharmacologic or behavioral therapy (7). Comorbid episodes of major depressive disorder frequently occur, especially in treatment-refractory cases of OCD (8). Patients with OCD plus comorbid major depressive disorder exhibit a cerebral metabolic substrate that differs from that of patients with pure OCD (9). The underlying mode of action by which anterior capsular electrostimulation suppresses the dysfunctional OCD circuitry is largely unknown. In this study, we investigated the functional metabolic effects of long-term stimulation of the anterior capsule using 18F-FDG PET. We also explored whether regional corticostriatothalamocortical metabolism was able to predict treatment outcome.
MATERIALS AND METHODS
Subjects
Six treatment-refractory OCD patients (2 men and 4 women; mean age ± SD, 40.4 ± 6.0 y) were entered consecutively into this study between 1998 and 2004. They were recruited at a single center and were evaluated by the same psychiatrist, who was unaware of treatment status. All met strict inclusion criteria, including the OCD criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (10), a Yale-Brown Obsessive Compulsive Scale score (Y-BOCS (11)) of greater than 30, and a Global Assessment of Functioning (12) score of less than 45 for a minimum of 5 y before study entry, despite multiple trials with serotonin reuptake inhibitors, clomipramine, augmentation strategies, and cognitive behavioral therapy (Table 1). All patients experienced comorbid depressive symptoms (Hamilton Depression Rating Scale (HAM-D (13) score > 16); at this stage of research, no pure OCD patients had undergone surgery.
Patient Profiles: Demographic Features and Clinical and Stimulation Characteristics
Quadripolar electrodes (Pisces Quad 388; Medtronic Inc.) were implanted in all patients as described in detail previously (4). In short, the stimulating contacts were placed in the internal capsule in a stereotactic procedure under general or local anesthesia depending on the patient's choice. Both electrodes were implanted symmetrically through precoronal burr holes in the internal capsule and nucleus accumbens. The electrodes were connected to 2 implanted pulse generators (Itrel II, Synergy, or Kinetra; Medtronic Inc.).
Simultaneous bilateral stimulation was applied to the anterior capsule in all but 1 patient (patient 4, Table 1), who received only right-sided stimulation because bilateral stimulation induced sadness. The stimulation parameters varied between a 210- and 450-μs pulse width and a 100- and 130-Hz frequency, and the voltage amplitude was ramped up to values between 4 V and the maximum tolerable level of 10.5 V, with an impedance of 700 Ω, to produce maximal clinical efficacy (decrease in OCD score).
Medication was minimized before the preoperative PET and was kept constant throughout the imaging study (Table 1).
Symptom severity before and after stimulation was assessed by 1 psychiatrist, using the Y-BOCS, HAM-D, and Beck Depression Inventory (BDI) (14) scores. These scores were registered at the time of each PET study, with a mean interval between evaluation and actual PET acquisition of 1.8 (±2.2) wk for the preoperative 18F-FDG studies and 0.5 (±1.7) wk for the stimulation 18F-FDG studies.
All patients gave witnessed oral and written informed consent. The study was approved by the local Ethics Committee and independently by the Commission for Neurosurgery for Mental Disorders in Flanders. For ethical reasons, no sham control studies were performed.
The metabolic results for the patient group were compared with 18F-FDG PET data from 20 age- and sex-matched healthy volunteers (9 men and 1 woman; mean age, 35.0 ± 8.9 y). These data were obtained from previous studies between 1998 and 2002 using the same scanning procedure and the same scanner. The healthy volunteers had no history of neurologic or psychiatric disorders and had normal findings on brain MRI.
PET Imaging Procedure
All patients who underwent electrode implantation also underwent 18F-FDG PET before and after the implantation. The preoperative PET study was conducted 14 ± 8 d, on average, before implantation (range, 2−28 d). The postoperative study in the stimulator-on condition was conducted on all patients 10.0 ± 8.3 mo, on average, after implantation (range, 3.0−26.5 mo). The stimulator-off condition was poorly tolerated by several patients and could be imaged in only 3 patients; that imaging took place 9.0 mo, on average, after stimulation onset (range, 7.9−10.5 mo). The periods during which the stimulator was switched off before PET acquisition were 1, 5, and 60 d. Because of this small number of patients and the very low sensitivity for the detection of significant changes, the results from the stimulator-off condition are not included in this report. One patient was excluded from the complete imaging study because of an associated tic disorder that caused severe movement artifacts on imaging.
18F-FDG was routinely produced in-house with radiochemical purity of more than 95% using a Cyclone 10/5 cyclotron (IBA). A 150- to 185-MBq injection of 18F-FDG was administered under standardized conditions (in a dimly lit, quiet room with the patient's eyes and ears open). Imaging data were acquired on a 3-dimensional HR+ PET camera (Siemens). Before emission scanning, a 10-min transmission scan was performed for attenuation correction using a 68Ge/68Ga source. This scan was followed 30 min later by a 30-min 3-dimensional static emission scan. Attenuation- and scatter-corrected reconstructed images were obtained by the 3-dimensional reprojection algorithm using the manufacturer's software and postreconstruction gaussian 2-dimensional filter smoothing (6 mm in full width at half maximum). All patients also underwent routine T1- and T2-weighted MRI (1.5 T, Vision; Siemens).
Data Analysis
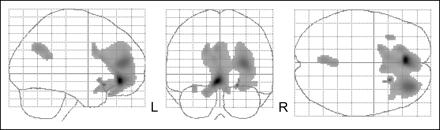
18F-FDG data were coregistered to each patient's MR images using statistical parametric mapping (SPM) (version 2; Wellcome Department of Imaging Neuroscience) and were spatially normalized using nonlinear warping with 16 iterations (voxel size after spatial normalization, 2 × 2 × 2 mm). Because of remaining inaccuracies in anatomic normalization in the subcortical nuclei (e.g., inaccuracies in the size, shape, and location of the caudate nucleus and in the location of the medial thalamic border because of third-ventricle enlargement in some patients), as shown in Figure 1, a parallel analysis was conducted using a template-based volume-of-interest (VOI) approach for subcortical regions, analogous to a previously described analysis (15). This template VOI was constructed by predefining all relevant subcortical VOIs on the spatially normalized, averaged patient magnetization-prepared rapid-acquisition gradient-echo MR image. VOIs were delineated for the whole gray matter on the basis of the atlas of Talairach and Tournoux (16). VOIs were constructed using the PMOD software VOI tool (PMOD Inc.). Six subcortical VOI pairs were selected: mediodorsal thalamic nucleus, putamen, caudate, ventral striatum, hippocampus, and amygdala. Individual adjustments based on the individual MR images were allowed for subcortical VOIs. For activity normalization, relative uptake values were determined by dividing the average VOI uptake by the total uptake over the whole of the brain gray matter (overall VOIs).
Inaccuracies in spatial normalization of homologous subcortical structures using 18F-FDG PET and SPM, version 2, with nonlinear warping (16 iterations) in 4 patients with OCD. Crossbars are centered on internal capsule between putamen and caudate head.
Statistical Analysis
For the 6 subcortical areas, VOI data were analyzed using Statistica, version 6.0 (StatSoft Inc.). Paired t statistics and correlation analyses were done parametrically. Results are given uncorrected for multiple comparisons because of the existing a priori hypotheses based on imaging findings in the neurocircuitry of OCD patients with comorbid depressive symptoms (15,17). One-sample t tests were used to compare the VOI uptake z scores of patients with those of healthy volunteers before and after stimulation under the null hypothesis that z score condition 2 minus z score condition 1 equals 0. Data reported for the 6 subcortical regions are thus based on VOI analysis. The threshold for significance for the subcortical VOI-related data was set at a P value of 0.01. Values of P lower than 0.05 are reported as indicating a trend toward significance.
SPM was used to screen the rest of the brain for nonhypothesized associations. Data were smoothed with an isotropic kernel of 10 mm. Proportional scaling was used to a value of 50, with an analysis threshold of 80%. To evaluate significant differences, we used an approach similar to that of Saxena et al. (15), with the peak threshold set at 0.001 (uncorrected) for cortical regions unless otherwise specified. The results were converted to the Talairach coordinates using a Montreal Neurological Institute (MNI)–to–Talairach conversion tool (18,19).
To characterize preoperative metabolism with respect to healthy volunteers, we performed a group comparison (2-sample t test). For the stimulation effects, a repeated-measures design (paired t test) was used. Furthermore, correlations were evaluated between preoperative metabolism and differential metabolism and between presurgical and stimulation conditions, with values of and differences in Y-BOCS, HAM-D, and BDI applied as covariates.
RESULTS
Preoperative Metabolism
Compared with healthy individuals, the patients showed a significantly reduced preoperative resting metabolism in the ventral prefrontal cortex and extending outward (more pronounced on the left side), bilaterally in the supplementary motor cortex and dorsal anterior cingulate, and in the left inferior parietal cortex (Brodmann's area [BA] 39) (P < 0.001) (Fig. 2). Metabolism was significantly increased in the cerebellum (P = 0.003) and left ventral striatum (P = 0.004). Trends toward increased metabolism were observed for the right ventral striatum (P = 0.03) and left amygdala (P = 0.02).
SPM glass brain representation of group metabolic characterization of refractory OCD patients with comorbid depressive symptoms. Large prefrontal cluster is seen extending to supplementary motor cortex. Image depicts peak threshold of 0.001 and extent threshold of 20 voxels.
Clinical and Metabolic Effects of Anterior Capsular Stimulation
Anterior capsular electrostimulation decreased Y-BOCS by a mean (±SD) of 13.5 ± 9.1 points (from 33.3 to 19.8; P = 0.01) and BDI by 16.7 ± 8.3 points (from 38.0 to 21.3; P = 0.004) at the time of PET (Fig. 3). The difference in HAM-D score was 10.2 ± 9.7 points (from 25.7 to 15.5; P = 0.05). As can be seen from Figure 3, in 1 patient the HAM-D score increased despite a clinically positive response on Y-BOCS.
Individual patient results for changes in clinical scores on chronic anterior capsular stimulation: Y-BOCS (A), HAM-D (B), and BDI (C). Horizontal lines are average values for groups. Preop = preoperative; Stim on = after chronic electrostimulation. *Overlapping data points.
Compared with the presurgical baseline, chronic anterior capsular electrostimulation induced a highly significant cluster of decreased activity in the subgenual anterior cingulate cortex (BA 32) dorsal to the posterior orbitofrontal cortex (peak MNI coordinates, x,y,z = −2,40,−8; z score = 4.79), with extension to the right dorsolateral prefrontal cortex and right anterior insula (Fig. 4; P < 0.001). Inverse SPM contrast (stimulator-on condition greater than preoperative condition) showed significant clusters bilaterally in the motor cortex (most pronounced on the right). Metabolism in the mediodorsal nucleus of the thalamus showed a trend toward decrease (P = 0.038 for the left nucleus and P = 0.048 for the right).
SPM glass brain representation of paired-group comparison between preoperative resting-state metabolism and stimulator-on condition in 6 refractory OCD patients.
In patients, anterior capsular electrostimulation normalized the preoperatively hyperactive ventral striatum and amygdala.
Correlation with Clinical Scores
A reduction in Y-BOCS scores correlated negatively with a decrease in metabolism in a subcortical network involving the left and right ventral striata (r = −0.87 and P = 0.02 for the left; r = −0.90 and P = 0.01 for the right). This is reflected in Figure 5, which shows the large extent to which the values of a single patient without clinical improvement influence this correlation. Negative correlations were also found between a reduction in Y-BOCS scores and a decrease in metabolism in the left hippocampus (r = −0.91; P = 0.01) and in the left posterior cingulate (BA 23) (r = −0.95; P = 0.005). The left amygdala (r = −0.91; P = 0.028) and left caudate head (r = −0.81; P = 0.05) showed a trend toward a decrease with reduction in Y-BOCS scores.
Correlation of ventral striatum metabolic activity with decrease in clinical Y-BOCS score. Circles and solid line are data for right side of brain; squares and dashed line are data for left side of brain. Decrease in score is score during stimulator-on condition minus score preoperatively.
The improvement in HAM-D depression severity correlated negatively with decrease in activity in the left hippocampus (r = −0.93; P = 0.007) and correlated positively with a decrease in metabolism in the right dorsolateral prefrontal cortex (r = 0.93 and P = 0.007 for BA 45; r = 0.92 and P = 0.009 for BA 46). No significant correlations were identified for BDI scores.
Prediction of Therapeutic Response
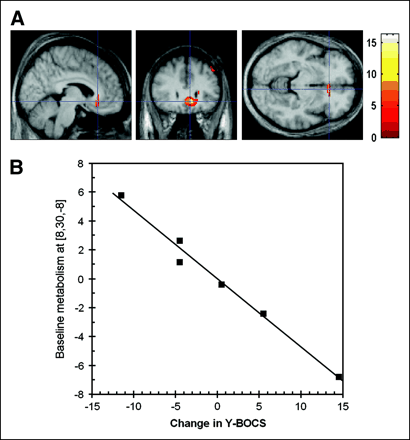
With regard to prediction of therapeutic response, SPM analysis showed that an improvement in Y-BOCS scores correlated significantly with (higher) preoperative activity in the subgenual anterior cingulate cortex (BA 25; peak MNI location, x,y,z = 8,30,−8; P = 0.001, uncorrected; z score = 3.94) (Fig. 6).
(A) Inverse correlation between preoperative metabolism and changes in Y-BOCS scores shows that subgenual activity centered at MNI coordinates (8,30,−8) is related to outcome of clinical treatment. Images are overlaid on average T1-weighted volumetric MRI scan of patient group. (B) Metabolic response at (8,30,−8) versus decrease in Y-BOCS score. Values are centered around mean change in Y-BOCS score (−13.5).
DISCUSSION
Apart from the use of 18F-FDG PET in anterior cingulotomy (20,21) and of perfusion SPECT in limbic leucotomy (22), to our knowledge no functional neuroimaging studies have previously been undertaken on neurosurgical approaches to OCD. This study is the first to describe the metabolic effects of anterior capsular electrostimulation in patients with refractory OCD.
Although OCD and major depressive disorder appear distinct in their course, prognosis, genetics, and neurochemical concomitants, the same corticostriatothalamocortical circuit is implicated in the pathogenesis of both disorders (23). Initial baseline studies of the use of 18F-FDG PET in OCD have found increased metabolic rates in the caudate nuclei and orbital gyri. Patients with pure OCD (drug-free, nondepressed patients) showed elevations of global cortical metabolism (24), including the caudate heads and orbital gyri. These results were confirmed by further baseline studies by Nordahl et al. (25) showing bilateral increases in the orbitofrontal cortex and by Swedo et al. (26) showing increases in the orbitofrontal cortex, right cerebellum, and thalamus, but also in the prefrontal cortex, when compared with healthy volunteers. The most comprehensive comparison of baseline 18F-FDG metabolism in OCD patients with and without symptoms of major depression was by Saxena et al. (9). These authors found that thalamic metabolism was significantly elevated in patients with OCD alone and with major depressive disorder alone. However, concurrent OCD and major depression showed another pattern, in which the thalamus, caudate, and hippocampus had lower metabolism than that in cases of OCD alone, suggesting that depressive episodes or comorbidity occurring in OCD patients may be mediated by different corticobasal ganglia-thalamic circuits. Also, in the study of Saxena et al., left hippocampal metabolism was reduced in patients with major depression and in patients with OCD and comorbid major depression, implicating involvement of the left hippocampus in both entities.
This study showed that, compared with healthy volunteers, patients had reduced prefrontal activity and increased activity in the ventral striatum and amygdala, confirming that the pattern of preoperative metabolism in this comorbid group is markedly different from the classic pattern in patients with pure OCD or major depressive disorder alone.
Neuroimaging data before and after treatment with paroxetine suggest that, with successful treatment, patients with OCD plus major depressive disorder exhibit striatal activity changes different from those of patients with OCD alone (27). In addition, the brain metabolic predictors of response to paroxetine described for OCD have been different from those described for major depressive disorder or for mixed forms (15), suggesting again that although both OCD and major depressive disorder respond to the same selective serotonin reuptake inhibitors, the two disorders have a different neurobiologic substrate for response.
The cognitive and motor deficits of major depressive disorder have been explained by a dysfunction within a dorsal compartment that includes the anterior, dorsal, and lateral prefrontal cortices; the dorsal anterior cingulate cortex; the parietal cortex; and the premotor cortex. The hypometabolic prefrontal and parietal disturbances found in this study are in line with these findings. On the other hand, the affective symptoms of major depressive disorder may be related to a dysfunction within a ventral compartment that includes the anterior cingulate, orbitofrontal, and anterior insular cortices. The ventral and dorsal compartments seem to be reciprocally inhibitory (7). The orbitofrontal cortex may be recruited in a compensatory fashion, because activity in this region correlates inversely with the symptom severity of major depressive disorder (28). Thus, the compensation of orbitofrontal cortex activity in OCD with major depressive disorder may result in average values in a group comparison.
The metabolic correlates of successful treatment imply a central role for the ventral striatum and subgenual anterior cingulate in the outcome of anterior capsular electrostimulation therapy for highly refractory OCD with comorbid depressive symptoms. The ventral striatum has a central position between the prefrontal, limbic, paralimbic, and striatal systems. It receives strong afferents from the basolateral amygdala, and its main efferents innervate the pallidum, striatum, mediodorsal thalamus, anterior cingulate cortex, and prefrontal and mesolimbic dopaminergic areas. We found hypermetabolism in the ventral striatum preoperatively and a negative correlation between changes in metabolism due to anterior capsular electrostimulation and improvements in clinical scores, indicating that ventral striatum hyperactivity needs to be preserved for a good clinical response in both depressive symptoms and obsessive-compulsive symptoms. In our series, the distal contact of the capsular electrodes was placed close to or in the ventral striatum (nucleus accumbens). These results are therefore not compatible with a hypothesis that direct inhibition of the ventral striatum is responsible for symptom improvement. Of note, in a pilot study, direct high-frequency deep-brain stimulation of the nucleus accumbens in OCD showed promising results (29).
To our knowledge, only a single previous study, restricted to patients with severe and treatment-resistant OCD, has been published. Rauch et al. (20) found that activity at a right posterior locus predicted the response to a procedure with a different anatomic target—anterior cingulotomy—but no resting-state comparison to a control group was described.
The subgenual anterior cingulate is a gateway region in the regulation of the ventral and dorsal frontal complex involved in mood and anxiety disorders. In capsulotomy studies, lesions of the ventral portion of the anterior limb of the anterior capsule are purported to interrupt orbitofrontal cortex/subgenual anterior cingulate cortex/thalamic connections. The placement of these lesions, however, might also interrupt frontostriatal projections. Our results show that for anterior capsular electrostimulation, deactivation of the subgenual anterior cingulate cortex or disruption of interconnections among the elements of the ventral compartment is a plausible mode of action (30). Increased activity in the subgenual anterior cingulate cortex was not observed preoperatively in this group—possibly in correspondence to the previously observed localized loss of glial cells, reduced glia, and smaller neurons (31). The importance of the subgenual anterior cingulate cortex is furthermore stressed by the predictive power of its metabolic activity as found in this study. Figure 6 shows that despite the few degrees of freedom, a highly significant linear correlation not influenced by outliers was found between presurgical subgenual activity and a Y-BOCS decrease. Such involvement of the subgenual anterior cingulate cortex is concordant with recent data on anterior cingulotomy (21). Interestingly, an increasing body of evidence shows that this region, which is associated with a decrease in OCD symptoms after deep-brain stimulation, is also consistently associated with responsiveness to antidepressant treatment in major depressive disorder (15,32–34).
The amygdala assess the reward and threat values of external stimuli and can drive the balance of activity toward the ventral compartment. The hippocampus, in addition to its role in cognition, has reciprocal connections with the amygdala and projects to the hypothalamus to influence the hypothalamus-pituitary-adrenal axis. Therefore, the possibility that amygdala hyperactivity and hippocampal inefficiency are central to the pathophysiology of major depressive disorder has been proposed. In our study, a more pronounced left-sided hypermetabolism of the amygdala was present, consistent with previous literature on major depressive disorder (35,36). Enlarged amygdala volumes have been found, although not consistently, in morphometric studies on patients with OCD (37) and patients with major depressive disorder (38).
With regard to methodology, 3 important issues need to be discussed. First, in this study we applied 2 different analytic approaches: a nonhypothesis-driven SPM approach and a hypothesis-driven predetermined-VOI approach. Such a combined approach increases the sensitivity of findings, especially subcortically, and has previously been applied successfully to similar pathologic conditions (15,39). Such a strategy is needed because of the difficulties in spatially normalizing data for small subcortical nuclei, especially the caudate, ventral striatum (nucleus accumbens), and thalamic nuclei, which form the central parts of the corticostriatothalamocortical circuit. This difficulty is demonstrated by Figure 1, which shows, for example, shifts between the centers of the caudate heads for different patients that are of the same order of magnitude as the dimensions of these structures.
Second, in contrast to many previous studies, we could define the ventral striatum and mediodorsal thalamus as separate entities. The sensitivity for detecting differences will be lower in these regions because of the partial-volume effect (40). Partial-volume correction was not performed, because existing methods are hampered by inaccurate MRI segmentation, especially in the subcortical areas. However, the observed effects in even the thalamic subnuclei, ventral striatum, and caudate are most likely underestimated because of the spillover effect from the surrounding ventricular and white-matter regions. Potential residual bias in the positioning of the subcortical VOIs may have been introduced, but such an effect should be minimal because pre- and postoperative data were spatially coregistered using rigid transformations.
Third, the limited sample size of the current study, and the fact that the applied thresholds were not fully corrected for multiple comparisons, imply a chance of false-positive findings (41). However, the threshold applied in the SPM analysis is one commonly used in the analysis of PET and SPECT studies with few degrees of freedom. For the VOI analysis, the threshold was set at a P value of less than 0.01 to reduce the incidence of false-positive findings to a level between that of an overly harsh Bonferroni correction (P = 0.05/12 regions) and that which would be (too) liberal, without any correction for multiple comparisons. However, we believed it worthwhile to mention as a trend those findings with P values of between 0.01 and 0.05, to indicate that these regions, located within the a priori defined corticostriatothalamocortical circuit, should be monitored closely in larger-scale investigations of metabolic correlations to anterior capsular stimulation in OCD.
CONCLUSION
This functional neuroimaging study, by showing substantial and specific dysfunction in the corticostriatothalamocortical neurocircuitry of patients with OCD and comorbid major depressive disorder, emphasizes the importance of the ventral striatum and subgenual anterior cingulate cortex. Confirmation of these pilot results in larger series may allow improved selection of targets and optimization of treatment.
Acknowledgments
We acknowledge the financial support of the Fund for Scientific Research, Flanders, Belgium (grants 1.5.236.99 and G.0273.97.N), the Research Council of the Katholieke Universiteit Leuven (grants OT-98-31 and OT-03-57), the Exploratory International Collaboration (Verkennende Internationale Samenwerking, VIS ZKB1159), Medtronic, Inc. (QUEST program L1170), and the University Hospital Leuven (a Clinical Research Mandate). We also acknowledge the OCD-DBS collaborative group for fruitful discussions.
Footnotes
-
↵* Contributed equally to this work.
References
- Received for publication September 2, 2005.
- Accepted for publication January 9, 2006.