Abstract
The therapeutic effects of peptide receptor–based radionuclide therapy are extensively being investigated in rats bearing tumors. Both the dose to the tumor and the therapy-limiting dose to normal tissues, such as kidneys and bone marrow, are of interest for these preclinical studies. The aim of this work was to develop a generalized computational model for internal dosimetry in rats. Methods: Mature rats were dissected and the relative positions, dimensions, and weights of all of their major organs were measured. A mathematic model was set up for the rat body and its internal organs to enable Monte Carlo radiation transport calculations to determine estimates for both tumor and organ self-doses as cross-organ doses for 90Y, 111In, and 177Lu. The organs and body were mostly of ellipsoid shape with the axes given as the measured length, width, and height normalized to values that, together with the measured weights, are consistent with the recommended soft-tissue and bone densities. A spheric tumor of 0.25 g was positioned on the right femur. Calculations were performed with the Monte Carlo neutral particle transport code MCNP for the β-emitters (maximum energy, 2.28 MeV) and 177Lu (maximum energy, 0.497 MeV) and for the γ-emissions from 177Lu and from 111In. The presented absorbed dose S values are used to calculate the absorbed dose estimates for the rat organs in a study on the biodistribution of 177Lu-DOTA-Tyr3-octreotate (DOTA is 1,4,7,10-tetraazadodecane-N,N′,N″,N‴-tetraacetic acid). Three activity distributions were considered in the kidney: uniform in the whole kidney, in the cortex, or in the outer 1-mm-thick rim of the cortex. Isodose curves and dose volume histograms were calculated for the dose distribution to the kidneys. Results: Depending on the activity distribution in the kidneys, the renal dose for 177Lu-DOTA-Tyr3-octreotate is 0.13–0.17 mGy/MBq. Conclusion: The renal dose of 70–95 Gy for an injected activity of 555 MBq will likely cause radiation damage, although the higher amount of peptide with this activity may influence the dosimetry by partial receptor saturation. Dose volume histograms show that 111In and 177Lu are likely to have a higher threshold for renal damage than 90Y.
- autoradiography
- radiobiology or dosimetry
- radionuclide therapy
- 111In
- 177Lu
- S factors
- 90Y
- rat dosimetry model
Scintigraphy with 111In-DTPA-octreotide (DTPA is diethylenetriaminepentaacetic acid) (Octreoscan; Mallinckrodt Medical, Inc.) has proven itself to be a very sensitive and specific method to localize somatostatin receptor–positive tumors and their metastases. Continuing research is aimed at developing a therapeutic analog, taking advantage of the specificity of the receptor binding and the localized radiation dose from the radionuclide linked to the peptide. As 111In emits 2 γ-rays, it is not optimal for therapy usage. Instead, 90Y-DOTA-Tyr3-octreotide (DOTA is 1,4,7,10-tetraazadodecane-N,N′,N″,N‴-tetraacetic acid), with the high-energy β-emitter 90Y (mean energy, 0.93 MeV; half-life [t1/2], 64 h) strongly linked in the DOTA-cage, has been developed and is now clinically being evaluated (1–3) for an optimized peptide receptor radionuclide therapy (PRRT). 90Y-DOTA-Tyr3-octreotide lacks γ-emission itself or a γ-ray–emitting diagnostic analog. Discrepancies between the renal uptakes of the positron-emitting analog 86Y-DOTA-Tyr3-octreotide and of 111In-DTPA-octreotide obscure the possibility of using the latter for dosimetry (R. Barone, written communication, November 2003 (4)). 177Lu (t1/2, 6.7 d) emits β-particles (mean energy, 0.13 MeV) as well as γ-rays suitable for imaging (113 keV at 6% per decay and 208 keV at 10% per decay). Together with a slightly altered somatostatin analog—octreotate, in which the amino acid threoninol at the C-terminal side of the octopeptide has been replaced by threonine—177Lu-DOTA-Tyr3-octreotate forms a superior therapeutic compound with considerably enhanced uptake in receptor-positive tumors (5,6).
The therapeutic effects of peptide receptor–based radionuclide therapy are extensively being investigated in studies with rats bearing tumors (7–10). It has been possible to observe tumor regression and, consequently, survival in a study of the effects of 177Lu-DOTA-Tyr3-octreotate in a rat model (7,9). Not only is the dose to the tumor of interest for these preclinical studies, but also is the therapy-limiting dose to normal tissues, such as kidneys and bone marrow. Using human dosimetry S values to estimate the dosimetry in the rat organs, as performed in the study by Lewis et al. (7), to explain observed toxicity introduces difficulties by the large difference in the dimensions from rat to human, as will be shown in this article.
Internal dosimetry for radionuclides depends on the dose estimation model used; in humans, the MIRD schema provides a generalized anatomic model with which the doses to all internal organs can be calculated from the organ residence times for the considered radionuclide (11). However, for the dosimetry of radionuclides applied in animals, no general models exist apart from the mouse model of Hui et al. (12), in which only 90Y dosimetry was considered. With the emphasis on the bone marrow dose for high-energy β-emitters, the authors concluded that low-energy β-emitters would also benefit from cross-organ radiation transport calculations. Dose volume histograms (DVHs) and 3-dimensional (3D) dosimetry for mouse liver, spleen, and kidney were introduced by Kolbert et al. (13). Suborgan dosimetry for mouse kidneys with differentiation of the cortex and medulla has been performed by Flynn et al. (14). These authors showed that the activity and dose distribution in the cortex is highly dependant on both the size of the antibody to which the activity is bound and to the range of the β-rays. The dose in the cortex can be >3 times larger than the dose in the medulla, and also within the cortex the dose may vary over 50%. A recently presented model by Hindorf et al. (15) for both mice and rats in a voxel-based geometry showed the relative insensitivity to organ shape (elliptic vs. spheric) and emphasized the importance of good interorgan positioning for dosimetry.
The aim of this work was to develop a generalized stylized calculational model for internal dosimetry of rats. Mature rats were dissected and the relative positions, dimensions, and weights of all of their major organs were measured. A mathematic model was set up for the rat body and its internal organs to enable Monte Carlo radiation transport calculations to determine estimates for both tumor and organ self-doses as cross-organ doses for 90Y, 111In, and 177Lu. The presented absorbed dose S values are used to calculate the absorbed doses for the rat organs in a study on the biodistribution of 177Lu-DOTA-Tyr3-octreotate (9).
MATERIALS AND METHODS
Three mature well-fed Wistar rats (average weight, 386 ± 35 g) were dissected and the dimensions and weights of the liver, spleen, kidneys, lungs, heart, stomach, small and large bowel, thyroid, femur and its bone marrow, testes, bladder, and carcass (Table 1) were measured. The relative positions of these organs within the rat body were based on the photograph of a dissected rat (Fig. 1) as well as on the topologic capability of fitting all organs within the rat outer body contour. The organs and body were modeled as ellipsoids with their axes given as the length, width, and height of each organ as measured within the dissected animals (Figs. 2 and 3). The skull, brain, spine, and spinal core dimensions were not actually measured but were estimated to a shape fitting inside the outer body contour. The dimensions were normalized to the measured organ weights to make them consistent with the recommended ICRU Report 46 soft-tissue and bone densities (16). To have a maximum cross-dose to the marrow cavity, a small spheric tumor of 0.25 g was positioned within the right femur. The defining mathematic equations and parameters used for the organs and body contour are listed in the Appendix.
Cut-open view of one of analyzed rats shows lungs, liver, pancreas, and bowel.
Visualization of stylized model of rat in MCNP4C with small tumor on hind leg.
Expanded view of tumor (green) on semitransparent femur shown with its marrow contents. All non-organ soft tissues and skin have been omitted.
Average Organ Dimensions of 3 Wistar Rats Weighing 386 ± 35 g
Calculations were performed with MCNP4C (17) for the β-emitters 90Y (maximum energy, 2.28 MeV) and 177Lu (maximum energy, 0.498 MeV) and for the Auger electron and γ-emissions from 111In and 177Lu. Specific β-spectra were obtained from the ICRP 38 database (18), the particle energy and emission probabilities for 177Lu were from Schötzig et al. (19), and the Auger electron spectrum for 111In was from Howell (20). The lower cutoff value for the energy for photons and β-rays was set at 0.01 MeV; for the low-energy Auger and internal conversion electrons, the threshold was set at 0.001 MeV. The number of electron histories needed to obtain acceptable absorbed energy fractions (φ) (statistical error < 5%) in adjacent organs is 500,000 for the β-rays from 90Y and 177Lu. The γ- and low-energy electron part of the 111In and 177Lu emission needed 1,000,000 histories. All source activities were distributed homogeneously over the source organs. The dosimetry to the femur bone was performed following Eckerman and Stabin (21). All bone marrow in the rat’s femur, however, is known to be active (red) marrow, unlike the human situation (21).
For the kidney uptake, selective uptake in the cortex and in an outer 1-mm-thick layer of the cortex was considered also. Autoradiography of the kidneys of rats injected with 177Lu-DOTA-octreotate showed selective uptake in the outer part of the cortex (9,22). The dimensions of the kidneys were therefore measured from these autoradiographs as well as the size of the cortex and the surface layer with the highest radioactivity uptake. In the kidneys, 3 distributions were analyzed: (a) homogeneous in the whole kidney; (b) homogeneous in the renal cortex; and (c) in the outside surface layer of the cortex, as shown in Figure 4. Four suborgan target regions were taken in the kidney: the renal cortex, the renal surface layer, the pelvis, and the whole kidney. As rat kidneys have a single lobe, the pelvis also includes the medulla. The dose distribution over the kidney was analyzed by separate Monte Carlo calculations for each activity distribution, using a 1-mm voxel-scoring grid over all kidney regions. By taking 106 electron and 2 × 106 photon histories, a compromise was made between the obtained accuracy in each voxel (∼8%) and the total calculation time (∼12 and 28 h on a Pentium III [Intel Corp.] personal computer, respectively). The dose distributions are visualized by isodose contours as well as by DVHs.
Cross-section through central planes of kidney model with 1-mm-thick surface (dark brown), rest of cortex (light brown), and pelvis region (gray). Adjacent organs are pancreas (light purple), liver (dark purple), small bowel (yellow), and spleen (light gray).
RESULTS
Absorbed fractions of energy emitted for the 0.25-g tumor model on one of the hind legs of the rat with incorporated 90Y, 111In, and 177Lu are given in Table 2. The Auger electrons of 111In and 177Lu are essentially all absorbed within the source organ itself. Only for the femur as a source organ does the self-dose for Auger electrons differ considerably from unity.
Absorbed Fractions of Emitted Energy (φ) in Rat Femur Tumor Model for 90Y, 111In, and 177Lu
For most organs, the β-part of the emissions from 177Lu is completely absorbed within the organ itself (φ ≥ 95%). The higher-energy β-rays from 90Y show a higher probability for escape from source organs and, therefore, will produce higher cross-organ doses. For instance, the energy absorption in the femur’s bone surface from activity in the tumor is 3.7% for 90Y and well below 1% for the other nuclides. The absorbed dose S values are calculated by converting MeV per decay to mGy·g/MBq·s and dividing by the target organ mass. The complete tables with S values are given in the Appendix. For the results of radioactivity uptake experiments with younger and smaller rats, the absorbed dose can be corrected by dividing by the organ mass ratio of these rats compared with the masses given in Table 1. Here, it is additionally assumed that corresponding changes in φ are not appreciable.
Kidney Model
Special attention was given to the effect of several activity distributions in the kidney; the results are given in Tables 3 and 4. For 90Y, the absorbed fraction in the whole kidney reduces from 71% to 55% when the activity distribution is changed from whole kidney to a surface source. The absorbed energy fraction in the cortex, however, barely shows a change between the 2 distributions: 48% versus 49%. With 177Lu, there is a 29% increase in the total S value to thecortex when the activity is distributed over the surface instead of the whole kidney, mainly due to the β-rays in the 177Lu emissions. For 111In, this increase is comparable: 25% and, despite the short range of its electrons, 5% of the emitted energy does not get absorbed in the cortex with a surface source distribution. The isodose curves for 90Y, 177Lu, and 111In in Figure 5 show these findings graphically. For 90Y, the inner boundary of the cortex aligns with the 80% isodose curve for the cortex activity distribution and with the 50% isodose curve for the surface activity distribution. In the case of 111In and 177Lu, this boundary corresponds with the 60% isodose curve for the cortex and with the 10% isodose curve for the surface distribution.
Isodose curves for 90Y, 111In, and 177Lu in longitudinal plane of kidneys for activity distributions: 1, kidneys; 2, renal cortex; 3, cortex surface layer of 1-mm thickness. The 5%, 10%, 20%, 40%, 60%, 80%, 100%, 120%, 140%, and 160% of average isodose curves are indicated.
Absorbed Fractions φ in Rat Kidney Model
Radiation Dose Estimates per Cumulated Activity S (Target ← Source) in mGy/MBq · s for 90Y and Different Radiation Components of 177Lu in Several Source Distributions Within Rat Kidney
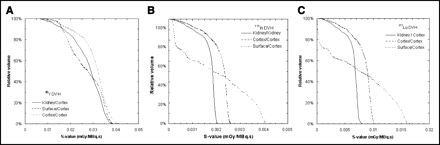
The volume distribution of the dose is best visible with the DVHs, as shown in Figure 6. A uniform dose will show an almost rectangular shape as seen with 111In and 177Lu for the whole kidney and cortex activity distributions. There is a slight falloff of the dose at the boundaries, but the dose in the renal cortex is, for these cases, well described by the average dose. For 90Y, the falloff is slightly larger, but already 16% of the cortex volume receives a dose below 0.024 mGy/MBq·s, or 25% below the average value in the case of the cortex distribution. When the activity is distributed in the outer 1-mm surface layer of the cortex, all nuclides show an irregular DVH. Not surprisingly, doses for 90Y appear to be less affected by the source distribution. 177Lu gets a very low dose (<6 × 10−4 mGy/MBq·s, or 7% of the average) in 25% of the cortex volume. With 111In, there is a lower threshold value by the longer-ranged γ-radiation, but still 25% of the cortex volume gets a dose below 3 × 10−4 mGy/MBq·s, or 13% of the average. For comparison with 90Y, the dose sparing at the inner cortex boundary yields a mere 4% of the cortex volume with a dose below 0.015 mGy/MBq·s, or 52% of the average. On the maximum side, 90Y also does not produce extremely higher doses for the surface distribution (10% cortex volume > 0.036 mGy/MBq·s, or 1.23 × average). For 111In and 177Lu, these values are much more extreme (for 111In: 10% > 0.0039 mGy/MBq·s, or 1.68 × average; for 177Lu: 10% > 0.015 mGy/MBq·s, or 1.75 × average).
DVHs for absorded dose to renal cortex for 90Y (A), 111In (B), and 177Lu (C) activity distributions as described in Figure 4.
Uptake of 177Lu-DOTA-Tyr3-Octreotate in Rats
The biodistribution of 177Lu-DOTA-Tyr3-octreotate was determined in tumor-bearing rats at several time intervals (9). 177Lu-DOTA-Tyr3-octreotate was intravenously injected at a specific activity of 3 MBq 177Lu per 0.5 μg peptide. The results are presented in Table 5. An exponential curve was fitted to the data, using the numeric option in the SAAM II code (SAAM Institute). The blood clearance showed a rapid clearance phase (99.9% with 24-min t1/2) and a very small redistribution phase (0.07% with 17-h t1/2 and 0.02% with infinite t1/2). The same fitting procedure was performed on the uptake data by Lewis et al. (7) and the results are shown in Table 5 for comparison.
Organ Masses, Residence Times (τ), and Dose per Injected Activity for 177Lu-DOTA-Tyr3-Octreotate in Rats According to Uptake Data of de Jong et al. and Lewis et al.
Mathematic Definitions and Parameters Used for Stylized Rat Model
Absorbed Dose S Values (in mGy/MBq · s) for 111In
Absorbed Dose S Values (in mGy/MBq · s) for 177Lu
Absorbed Dose S Values (in mGy/MBq · s) for 90Y
With escalation of the injected activities to levels higher than considered in this dosimetry study, the increased mass of peptide administered (assuming constant specific activity) will cause lower uptake in receptor-positive organs. Partial saturation of the receptors is causing this effect, which has been shown to potentially lower the uptake in the tumor with 50% (9,23). The dosimetry values for the receptor-positive organs in rats (adrenals, pancreas, pituitary, and stomach) and tumors are only valid at the peptide mass used in this study.
The injected activities to the rats were escalated to 555 MBq, leading to a kidney dose of 72 and 49 Gy, respectively, for both datasets. If the activity is taken up in the cortex, the dose to the cortex goes up to 95 and 64 Gy, respectively. For an activity uptake just in the outer 1-mm surface layer of the cortex, the dose in this surface layer is 172 Gy and the mean dose to the cortex becomes 92 Gy (115 and 63 Gy (7)). It can be derived from the DVHs that for a surface distribution the dose in the cortex varies between 5 Gy (12% volume) and 165 Gy (5% volume). The other distributions show a D05 (5% of the volume is exceeding this dose) comparable to the mean cortex dose: D05(cortex) = 104 Gy and D05(kidney) = 79 Gy. In external-beam radiotherapy, the effective volume method is used to characterize nonuniformly irradiated normal organs (24,25); this method yields the following effective volumes: Veff(kidney) = 74%, Veff(cortex) = 72%, and Veff(surface) = 40%.
DISCUSSION
Normal tissue radiation toxicity in rats from radiolabeled compounds can now be compared on a dose level (in Gy) instead of injected activities (in MBq). The advantage of such an approach is apparent; it clears the uncertainty in upscaling from injected activities in rats to the equivalent in humans (26). However, the influence of high mass amounts of peptides on the biodistribution, when administering activities at normal organ radiation toxicity levels in rat experiments, limits the use of the dose values for receptor-positive organs determined at lower peptide concentrations (23). Fortunately, the uptake in the kidneys is not receptor mediated. Both the effects as well as the use of human dosimetry in the evaluation of dose effects in rats by 177Lu-DOTA-Tyr3-octreotate obscure the conclusions of the otherwise excellent work by Lewis et al. (7). For instance, their kidney dose estimate of 0.670 ± 0.047 mGy/MBq does not indicate any probability of finding radiation damage in the kidneys with an injected activity of 555 MBq, leading to a minimal renal dose of 0.37 Gy. Using the rat-based dosimetry of this article, the dose of 49 Gy for a uniform distribution over the kidneys is more in the range where renal damage for fractionated radiation can be expected (27). The doses in the range of 63–64 Gy by a more localized activity distribution will likely cause observable effects in the kidneys.
Evaluation of the effects by a nonuniform radiation distribution in the kidneys by PRRT cannot be directly inferred from the extensive experience in radiobiology with external beams. With PRRT the distribution is related to physiologic uptake in the kidneys, whereas for x-ray irradiation anatomic contours and external-beam modifiers define the distribution. The effective volume method assumes that the functional units are uniformly distributed over the considered volume. It is still uncertain for rat kidneys whether the glomeruli, which form the radiation-sensitive functional units for late damage, are evenly distributed over the cortex, whereas in human kidneys the majority of the glomeruli are placed in the outer cortical regions (28).
Behr and Béhé (29) observed a remarkable difference in toxicity in preclinical therapy experiments with DTPA-d-Glu1-minigastrin labeled with either 90Y or 111In in tumor-bearing mice. 90Y induced chronic nephropathy at a renal dose of ≥60 Gy, whereas renal damage for 111In was found at approximately twice this dose. Dosimetry for 90Y may be influenced by the large difference in volume of a mouse kidney (0.15–0.18 cm3 (13)) and a rat kidney (1.6 cm3), by both differences in φ (52% vs. 71%) as well as differences in the radiation distributions. The much lower range of the 111In Auger electrons, however, will yield quite comparable radiation distribution patterns in mouse and rat kidneys. The findings by Behr and Béhé can be explained by the DVHs. For an average kidney dose of 60 Gy the DVHs, with 90Y and 111In distributed in the outer cortex surface, are shown in Figure 7. With 111In 45% of the cortical volume gets a dose of <10 Gy, whereas for 90Y the cortex dose exceeds 20 Gy almost (99%) everywhere. It is currently not known whether minigastrin shows the same uptake pattern in the kidneys as found for octreotate.
DVH for renal cortex dose by activities of 90Y and 111In that produce average dose to kidneys of 60 Gy, when uniformly distributed in kidneys. Activity distribution is over renal cortex surface.
CONCLUSION
A general-purpose stylized model rat is presented, for which the S factors for 90Y, 111In, and 177Lu have been calculated. Dosimetry was performed for a biodistribution experiment with 177Lu-DOTA-octreotate. DVHs and isodose lines were calculated for 3 types of radioactivity distributions within the kidneys, showing a large deviation from a uniform distribution to a more realistic distribution in the supracortical region of the kidneys. With the distribution pattern for octreotate renal uptake, DVHs show that 111In and 177Lu are likely to have a higher threshold for renal damage than 90Y, like the experience with minigastrin (29).
APPENDIX
The defining mathematic equations and parameters used for the organs and body contour are given in Table A1. The absorbed dose S values for 111In, 177Lu, and 90Y are given in Tables A2, A3, and A4, respectively.
Footnotes
Received Nov. 3, 2003; revision accepted Jan. 29, 2004.
For correspondence or reprints contact: Mark W. Konijnenberg, PhD, Mallinckrodt Medical BV, P.O. Box 3, 1755 ZG Petten, The Netherlands.
E-mail: mark.konijnenberg{at}emea.tycohealthcare.com