Abstract
111In-nuclear localization sequence-trastuzumab is a radioimmunotherapeutic agent consisting of trastuzumab modified with NLS peptides (CGYGPKKKRKVGG) and labeled with the Auger electron emitter 111In. Our objectives were to evaluate the tumor growth–inhibitory properties and normal-tissue toxicity of 111In-NLS-trastuzumab in mice after intraperitoneal administration. Methods: The pharmacokinetics of 111In-NLS-trastuzumab after intravenous (tail vein) or intraperitoneal injection in BALB/c mice were compared. Normal-tissue toxicity was determined in BALB/c mice at 2 wk after intraperitoneal injection of 3.7–18.5 MBq (4 mg/kg) of 111In-NLS-trastuzumab by monitoring body weight, histopathologic examination of tissues, and hematology (white blood cell, platelet, red blood cell, and hemoglobin) or clinical biochemistry (alanine transaminase and creatinine) parameters. A no-observable-adverse-effect-level (NOAEL) dose was defined. Athymic mice bearing subcutaneous MDA-MB-361 or MDA-MB-231 human breast cancer xenografts (5.0 × 105 or 0.5 × 105 HER2/cell, respectively) were treated with a single NOAEL dose or 2 doses administered intraperitoneally and separated by 2 wk. Control groups were administered 111In-trastuzumab, trastuzumab, nonspecific 111In-NLS-human IgG (hIgG), or normal saline. Results: The bioavailability of 111In-NLS-trastuzumab after intraperitoneal injection was 0.7. The NOAEL dose was 9.25 MBq (4 mg/kg); doses greater than or equal to 18.5 MBq decreased white blood cell or platelet counts, and doses of 27.7 MBq decreased red blood cell counts. There was no increase in alanine transaminase or creatinine at any doses tested. There were no morphologic changes to the liver, kidneys, heart, or spleen or loss of body weight. A single dose of 111In-NLS-trastuzumab (9.25 MBq)—compared with mice receiving 111In-trastuzumab, trastuzumab, 111In-NLS-hIgG, or normal saline—significantly slowed the rate of growth of MDA-MB-361 tumors over 60 d (0.014 d−1 vs. 0.033 d−1, 0.046 d−1, 0.030 d−1, and 0.061 d−1, respectively; P < 0.05). 111In-NLS-trastuzumab had no effect on the growth of MDA-MB-231 tumors. Two doses of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg) separated by 2 wk increased the survival of mice with MDA-MB-361 tumors, compared with mice treated with trastuzumab or normal saline (>140 d vs. 96 and 84 d, respectively; P < 0.001 or 0.027, respectively). Conclusion: 111In-NLS-trastuzumab is a promising radioimmunotherapeutic agent that could be effective for treatment of HER2-overexpressing breast cancer in humans.
The humanized monoclonal antibody trastuzumab (Herceptin; Hoffmann-LaRoche) is among the first target-specific drugs to be developed for immunotherapy of HER2-amplified breast cancer (1). In the clinical setting, trastuzumab has been shown to prolong survival in women with HER2-overexpressing metastatic breast cancer when used alone or with chemotherapy (2). However, only 30%−50% of patients with disseminated disease respond to trastuzumab combined with chemotherapy, despite having tumors with amplified HER2 (3,4). Moreover, the emergence of resistance in responding patients remains challenging (5). Nonetheless, improvements in overall and disease-free survival have been reported from several large clinical trials in which women with early-stage breast cancer received adjuvant trastuzumab therapy regimens (6).
Our group has been exploring the possibility of enhancing the efficacy of HER2-targeted immunotherapy of breast cancer by combining trastuzumab with an Auger electron–emitting radionuclide, 111In. Auger electrons are low-energy (<30 keV) electrons that deposit all their energy over nanometer to at most micrometer distances in tissues. The decay of an Auger electron emitter inside a cell can cause severe DNA double-strand breaks and cell death (7). However, to fully exploit this phenomenon, the radionuclide must be delivered to the tumor cell nucleus, where these extremely short-range electrons are especially damaging to DNA and lethal (8). Therefore, we further modified 111In-labeled trastuzumab with 13-mer peptides (CGYGPKKKRKVGG) harboring the nuclear localization sequence (NLS) of the simian virus 40 large T-antigen. NLS conjugation to 111In-trastuzumab enhanced its nuclear uptake and cytotoxicity toward HER2-overexpressing human breast cancer cells in vitro (9). Moreover, the NLS-peptide modifications did not substantially interfere with the receptor-binding ability in vitro or the tumor localization properties of 111In-trastuzumab in vivo but did promote HER2-specific nuclear uptake in mice bearing MDA-MB-361 xenografts (9). We subsequently reported that 111In-NLS-trastuzumab could kill breast cancer cells with acquired trastuzumab resistance, thereby illustrating that the radiopharmaceutical may have the potential to target both trastuzumab-sensitive and trastuzumab-resistant breast cancer metastases with HER2 overexpression (10).
In the current study, our goal was to evaluate the pharmacokinetics, normal-tissue toxicity, and antitumor effects of 111In-NLS-trastuzumab administered to mice implanted subcutaneously with human breast cancer xenografts with high or low HER2 expression. Bone marrow toxicity is generally dose-limiting for most radiotherapeutic agents (11). However, the use of radiopharmaceuticals labeled with 111In that emit nanometer- to micrometer-range Auger electrons should restrict killing mostly to targeted breast cancer cells and minimize toxicity toward nontargeted cells, such as hematopoietic stem cells (which express minimal levels of HER2) (12). We hypothesized, therefore, that 111In-NLS-trastuzumab would exhibit potent and selective tumor growth–inhibitory effects on HER2-overexpressing breast cancer xenografts in vivo while causing low normal-tissue toxicity.
MATERIALS AND METHODS
Cell Culture
MDA-MB-361 and MDA-MB-231 human breast cancer cells lines were obtained from the American Type Culture Collection. MDA-MB-361 cells were cultured in Leibovitz (L-15) cell culture medium (Invitrogen) supplemented with 20% fetal bovine serum (Sigma-Aldrich), and MDA-MB-231 cells were cultured in Dulbecco's minimal essential medium (Ontario Cancer Institute) supplemented with 10% fetal bovine serum and 100 U of penicillin per milliliter and 100 μg of streptomycin per milliliter.
111In-NLS-Trastuzumab and 111In-NLS-Human IgG (hIgG)
Trastuzumab or nonspecific hIgG (Product no. I4506; Sigma-Aldrich) was conjugated to diethylenetriaminepentaacetic acid (DTPA) dianhydride (Sigma-Aldrich) and sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Pierce) for subsequent reaction with synthetic 13-mer NLS-peptides (CGYGPKKKRKVGG) and labeling with 111In, as described previously (9). Briefly, trastuzumab or hIgG (500 μg, 10 mg/mL) was reacted with a 10-fold molar excess of DTPA dianhydride before reaction with a 15-fold molar excess of sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (2–5 mmol/L). Maleimide-derivatized and DTPA-modified trastuzumab or hIgG were then reacted overnight at 4°C, with a 60-fold molar excess of NLS-peptides (5–10 mmol/L in phosphate-buffered saline, pH 7.0). NLS-modified and DTPA-conjugated trastuzumab or hIgG were purified on a Sephadex-G50 minicolumn eluted with phosphate-buffered saline (pH 7.0). We previously determined that under these conditions, 3–4 NLS-peptides were conjugated to trastuzumab at succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate:IgG:NLS-peptide molar ratios of 15:1:60 (9).
The NLS-modified trastuzumab or NLS-hIgG DTPA-immunoconjugates or unmodified DTPA-conjugated trastuzumab were radiolabeled by incubation of 250–500 μg with 37–111 MBq of 111InCl3 (MDS-Nordion) for 1 h at room temperature. 111In-labeled proteins were purified using a Sephadex-G50 minicolumn eluted with phosphate-buffered saline (pH 7.0) and by passage through a Microcon YM-50 ultrafiltration device (Millipore). The radiochemical purity was routinely greater than 97%, as determined by instant thin-layer silica-gel chromatography (Pall Corp.) developed in sodium citrate (100 mmol/L, pH 5.0). We previously reported that the proportion of monomers to higher-molecular-weight dimer- and polymer-radiolabeled IgG complexes was greater than 85%, and the dissociation constant for binding of 111In-NLS-trastuzumab, compared with that of 111In-trastuzumab, to HER2-positive SK-BR-3 cells was reduced less than 3-fold (9).
Pharmacokinetics of 111In-NLS-Trastuzumab After Intravenous or Intraperitoneal Injection
For radioimmunotherapy studies, it was most practical to administer 111In-NLS-trastuzumab by intraperitoneal injection. Therefore, the pharmacokinetics and bioavailability of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg) administered by intraperitoneal or intravenous (tail vein) injection were first compared in non–tumor-bearing BALB/c mice. Blood samples (5–10 μL) were collected in heparinized microcapillary tubes (Fisher Scientific) at selected times between 5 and 30 min and 1 and 72 h after radiopharmaceutical injection by nicking the saphenous vein with a sterile scalpel blade. Radioactivity in the blood samples was measured in a γ-counter, and the concentration of 111In was expressed as counts per minute per microliter of blood. The blood radioactivity–time curve for the intravenous route was fitted to a 2-compartment pharmacokinetic model, and the curve for intraperitoneal administration was fitted to a 1-compartment model with an absorption phase, using Prism software (version 4.0; GraphPad). Pharmacokinetic parameters calculated included distribution half-life, elimination half-life, absorption half-life (determined for intraperitoneal administration only), volume of distribution of the central compartment (not determined for intraperitoneal administration), volume of distribution at steady state (Vss), systemic clearance, and the area under the curve. The area under the curve from the intraperitoneal curve was divided by the area under the curve from the intravenous curve to calculate the bioavailability, which was defined as the fraction of radiopharmaceutical that reached the systemic circulation after an intraperitoneal injection. All animal studies were approved by the Animal Care Committee at the University Health Network (protocol 282.6) following the Canadian Council on Animal Care guidelines.
Normal-Tissue Toxicity of 111In-NLS-Trastuzumab
To examine the normal-tissue toxicity of 111In-NLS-trastuzumab, groups of non–tumor-bearing female BALB/c mice were injected intraperitoneally with a single dose or 2 doses of 111In-NLS-trastuzumab separated by 2 wk (3.7–27.7 MBq; 4 mg/kg) in 150 μL of sodium chloride (150 mmol/L; normal saline). Control mice received an intraperitoneal injection of a single dose of 111In-NLS-hIgG (18.5 MBq; 4 mg/kg) or the equivalent volume of normal saline vehicle. Hematologic toxicity was monitored by obtaining peripheral blood cell counts at the end of a 2-, 3-, 5-, or 7-wk observation period after the final dose of the radiopharmaceutical. Blood was removed via cardiac puncture, and 250–500 μL were collected into ethylenediaminetetraacetic acid–coated tubes (Becton-Dickinson). Hematology analysis included white blood cell (WBC) counts, erythrocyte red blood cell (RBC) counts, platelet counts, and hemoglobin concentrations, all of which were measured at the Clinical Biochemistry Laboratory at the Hospital for Sick Children. Normal tissues were removed, sectioned, and stained with hematoxylin and eosin for histopathologic examination by light microscopy for any morphologic evidence of acute toxicity. Kidney sections were also stained with periodic acid–Schiff stain to reveal the extracellular matrix. All sections were evaluated by a clinical pathologist.
The potential for acute hepatotoxicity and renal toxicity from 111In-NLS-trastuzumab was further assessed by measuring serum alanine transaminase (ALT) and creatinine levels at 72 h after intraperitoneal injection of 111In-NLS-trastuzumab. Blood was collected in microtubes and allowed to clot at room temperature for 15 min, followed by centrifugation at 1,000g for 10 min at 4°C to isolate the serum. No hemolysis was visible in any serum samples. Serum creatinine was measured using a picric acid–based colorimetric assay kit (Infinity Creatinine; Thermo Electron). Serum ALT levels were measured with Infinity ALT reagent (Thermo Electron), using the rates of change in absorbance at 340 nm and the absorption coefficient for nicotinamide adenine dinucleotide (6.22 cm−1 mM−1) to calculate the enzyme activities.
Radioimmunotherapy Studies
The tumor growth–inhibitory properties of trastuzumab, 111In-trastuzumab, 111In-NLS-trastuzumab, and 111In-NLS-hIgG were compared in female athymic CD-1 mice implanted subcutaneously with MDA-MB-361 (5.0 × 105 HER2 receptors per cell (13)) or MDA-MB-231 (0.5 × 105 HER2 receptors per cell (13)) human breast cancer xenografts. To establish the tumors, mice were implanted intradermally with a 17-β-estradiol pellet (Innovative Research of America) 24 h before inoculation in the flank with 1 × 107 MDA-MB-361 cells or 1 × 106 MDA-MB-231 cells in 100 μL of a mixture of 1:1 (v/v) of Matrigel (Becton-Dickinson) and culture medium. The mice received an intraperitoneal injection of 9.25 MBq of 111In-trastuzumab, 111In-NLS-trastuzumab, or 111In-NLS-hIgG when the tumors reached a diameter of 2–5 mm (6–8 wk) on day 0 for the single-dose study or on days 0 and 14 for the 2-dose study. The administered IgG dose was 4 mg/kg, or approximately 80 μg for a mouse weighing 20 g. This dose is comparable to that in the clinical setting in which trastuzumab is administered as a loading dose of 4 mg/kg, followed by weekly doses of 2 mg/kg (14). The tumor length and width were measured using calipers, and the body weights were monitored every 7–14 d for 8 wk. The tumor volume was calculated as volume = (length × width2) × 0.5, and the tumor growth index (TGI) was calculated by dividing the tumor volume at each time point by the initial tumor volume. Similarly, body weight indices (BWIs) were calculated by dividing the body weight at each time point by the pretreatment body weight. The mean TGI and BWI were plotted versus the time from the start of treatment to obtain tumor growth and body weight curves. Treatment experiments were terminated when tumor size exceeded a mean diameter of 12 mm or at the planned end of the study (60 and 140 d for the single-dose and 2-dose studies, respectively). The median survival time was determined to be the time at which half of the animals were either sacrificed (because the endpoint had been reached) or died during the evaluation period.
Statistical Methods
Data were presented as mean ± SEM. Significance was evaluated by 1-way ANOVA, followed by the Tukey post hoc test using Prism software (version 4.0; GraphPad). The rate of tumor growth in animals treated with 111In-NLS-trastuzumab or in control animals was determined from the slope of the TGI-versus-time curve fitted by linear regression analysis. Statistical comparisons of slopes were made using the F test. Survival curves were constructed using the method of Kaplan–Meier. Comparisons among different treatment groups were performed using the log rank test. A P value of less than 0.05 was considered significant.
RESULTS
Pharmacokinetics of 111In-NLS-Trastuzumab After Intravenous or Intraperitoneal Injection
The elimination of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg) after intravenous injection (data not shown) was described by a 2-compartment pharmacokinetic model. The α-phase (distribution) half-life was 0.14 h, and the β-phase (elimination) half-life was 33.7 h (Table 1). The volume of distribution of the central compartment and the Vss for 111In-NLS-trastuzumab after intravenous injection were 1.1 mL (55 mL/kg) and 1.7 mL (85 mL/kg), respectively. The pharmacokinetics of 111In-NLS-trastuzumab after an intraperitoneal injection was described by a 1-compartment model with an absorption and elimination phase. The bioavailability of 111In-NLS-trastuzumab after intraperitoneal administration was 0.7 (i.e., 70% reached the systemic circulation), and the time to maximum blood concentration was reached by 6.9 h (Table 1). The Vss of 111In-NLS-trastuzumab after intraperitoneal injection was 1.2 mL (60 mL/kg), slightly lower than the Vss when the radiopharmaceutical was administered by intravenous injection.
Pharmacokinetic Parameters for 111In-NLS-Trastuzumab Administered Intravenously or Intraperitoneally to Non–Tumor-Bearing BALB/c Mice
Normal-Tissue Toxicity of 111In-NLS-Trastuzumab
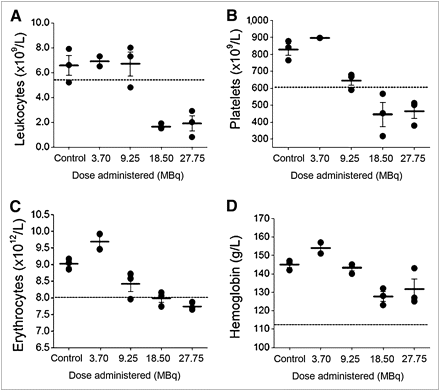
There were no observable adverse effects on the hematopoietic system of non–tumor-bearing normal BALB/c mice at 2 wk after intraperitoneal injection of 111In-NLS-trastuzumab at single doses of 9.25 MBq or less (4 mg/kg; Fig. 1). The no-observable-adverse-effect level (NOAEL) was thus defined as the dose that did not cause any significant change in blood counts, compared with normal values or those of control, normal saline–treated mice. Compared with saline-treated mice, 111In-NLS-trastuzumab–treated mice showed a significant decrease in WBC and platelet counts for doses of 18.5 MBq or greater (Figs. 1A and 1B), and these parameters were below the normal lower limit for mice (15). The RBC count also fell just slightly below the normal lower limit but only at the highest administered dose (27.7 MBq; 4 mg/kg), whereas hemoglobin levels decreased but did not fall below the normal limit after administration of 111In-NLS-trastuzumab (Figs. 1C and 1D). In mice administered 18.5 MBq (4 mg/kg) of 111In-NLS-trastuzumab, platelet counts returned to normal at 5 wk or later (Fig. 2). However, WBC counts remained below the reference range up to 7 wk after injection of 18.5 MBq (4 mg/kg) of 111In-NLS-trastuzumab, although there was a trend toward increasing counts (Fig. 2). Similarly, WBC counts were suppressed to a greater extent than platelet counts (3.23 ± 0.21 × 109/L and 567.7 ± 63.5 × 109/L, respectively; data not shown) at 2 wk after intraperitoneal injection of 18.5 MBq of 111In-NLS-hIgG. Peripheral blood counts were further assessed in normal BALB/c mice that received 2 intraperitoneal injections of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg each). At 2 wk after the second injection, the hematologic parameters from each mouse remained above the lower normal limit, and the mean WBC, platelet, and RBC counts and hemoglobin concentrations were 5.72 ± 0.21 × 109/L, 711.5 ± 38.1 × 109/L, 7.66 ± 0.13 × 1012/L, and 128.5 ± 2.62 g/L, respectively.
Leukocyte counts (A), platelet counts (B), erythrocyte counts (C), and hemoglobin concentrations (D) in blood of non–tumor-bearing normal BALB/c mice 2 wk after intraperitoneal injection of increasing single doses of 111In-NLS-trastuzumab or normal saline (control). Broken line indicates normal lower limit of measured parameter.
Leukocyte (A) and platelet (B) counts in blood of non–tumor-bearing normal BALB/c mice measured at 3, 5, and 7 wk after intraperitoneal injection of single dose of 18.5 MBq (4 mg/kg) of 111In-NLS-trastuzumab. Broken line indicates normal lower limit of measured parameter.
Serum ALT and creatinine levels remained within the reference range (15) for non–tumor-bearing normal BALB/c mice at 72 h after intraperitoneal injection of increasing single doses of 111In-NLS-trastuzumab (3.7–27.7 MBq; 4 mg/kg; Fig. 3). Serum ALT levels ranged from 23 to 55 units/L for 111In-NLS-trastuzumab–treated mice and from 20 to 42 units/L for control, normal saline–treated mice. The serum creatinine concentrations ranged from 40 to 68 μmol/L for 111In-NLS-trastuzumab–treated mice and 30 to 53 μmol/L for saline-treated mice. These results indicated no acute toxicity to the liver or kidneys from 111In-NLS-trastuzumab at doses up to 3 times higher than the NOAEL.
Serum ALT (A) and creatinine (B) levels at 72 h after intraperitoneal injection of increasing doses of 111In-NLS-trastuzumab or normal saline (control) in non–tumor-bearing normal BALB/c mice. Cr = creatinine.
The results of histopathologic examination by hematoxylin and eosin staining revealed no morphologic changes to the brain, heart, lung, stomach, small intestine, large intestine, liver, kidney, spleen, and muscle tissues (data not shown). In particular, no changes characteristic of hepatotoxicity or renal toxicity were observed. Periodic acid–Schiff staining of the renal glomeruli did not reveal basement membrane thickening, and the mesangial matrix was otherwise unremarkable (data not shown).
Radioimmunotherapy Studies
Radioimmunotherapy studies were conducted in athymic mice bearing subcutaneous MDA-MB-361 tumors overexpressing HER2 or MDA-MB-231 tumors with a 10-fold lower HER2 density (5.0 × 105 vs. 0.5 × 105 receptors per cell, respectively) (13). In 1 experiment, tumor-bearing mice received a single intraperitoneal injection of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg) or the equivalent amount of 111In-trastuzumab, 111In-NLS-hIgG, or unlabeled trastuzumab. Control mice were treated with a single intraperitoneal injection of normal saline. The average initial tumor volume in each treatment and control group ranged between 41.6 ± 4.9 and 51.7 ± 7.4 mm3 (P > 0.05, ANOVA). Linear regression analysis of the tumor growth (TGI) curves revealed that the proliferation rate of MDA-MB-361 tumors was slowest in mice treated with 111In-NLS-trastuzumab and that this rate was 4-fold less than the rate of tumor growth in normal saline–treated mice (slopes of tumor growth curves, 0.014 d−1 vs. 0.061 d−1, respectively; F test; P < 0.05) (Fig. 4A). The tumor growth rate was also significantly lower in mice treated with 111In-NLS-trastuzumab than that in mice treated with 111In-trastuzumab, trastuzumab, or 111In-NLS-hIgG (slopes, 0.033 d−1, 0.046 d−1, and 0.030 d−1, respectively; F test; P < 0.05). In contrast, a single dose of 111In-NLS-trastuzumab had no effect on the growth of MDA-MB-231 xenografts when compared with unlabeled trastuzumab- or normal saline–treated groups (slopes, 0.150 d−1 vs. 0.144 d−1 and 0.151 d−1, respectively) (Fig. 4C). Analysis of the BWI curves over the 60-d observation period revealed no significant differences between the 111In-NLS-trastuzumab–treated and control groups, with all mice gaining weight. This analysis suggests no generalized toxicity from the radiopharmaceutical in these athymic tumor-bearing mice at the NOAEL predetermined in non–tumor-bearing normal BALB/c mice (Figs. 4B and 4D).
TGI vs. time and BWI vs. time for athymic mice implanted subcutaneously with HER2-overexpressing MDA-MB-361 (A and B, respectively) or low-HER2-expressing MDA-MB-231 (C and D, respectively) human breast cancer xenografts. Data were obtained after treatment with single intraperitoneal injection of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg). Control groups were administered equivalent doses of trastuzumab, 111In-trastuzumab, 111In-NLS-hIgG, or normal saline. Treatments were started on day 0. Points represent mean ± SEM (n = 6–7 mice per group).
In a second experiment, groups of mice bearing subcutaneous MDA-MB-361 tumors were administered (separated by 2 wk) 2 single doses of 111In-NLS-trastuzumab (9.25 MBq, 4 mg/kg) or the equivalent doses of unlabeled trastuzumab. Mice that received 111In-NLS-trastuzumab had a median survival time of more than 140 d, which was significantly greater than the survival times of mice treated with unlabeled trastuzumab or normal saline (median survival, 96 and 84 d, respectively) (Fig. 5; P = 0.0008 or 0.027 for 111In-NLS-trastuzumab vs. saline or trastuzumab treatments, respectively). Moreover, complete tumor regressions (i.e., absence of a measurable tumor xenograft) were achieved in 3 of 6 mice treated with 111In-NLS-trastuzumab but in only 1 of 6 mice treated with trastuzumab and 0 of 6 mice administered normal saline. Analysis of BWI curves over the 140-d observation period revealed no significant differences between the 111In-NLS-trastuzumab–treated and control groups (data not shown).
Kaplan–Meier survival curves of athymic mice bearing HER2-positive MDA-MB-361 human breast cancer xenografts and administered 2 doses each of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg), trastuzumab (4 mg/kg), or normal saline via intraperitoneal injection on days 0 and 14 (n = 6 mice per group).
DISCUSSION
Our results revealed that trastuzumab conjugated to NLS-peptides and labeled with the Auger electron–emitting radionuclide 111In administered at the NOAEL (9.25 MBq; 4 mg/kg) strongly inhibited the growth of HER2-overexpressing subcutaneous MDA-MB-361 human breast cancer xenografts in athymic mice. However, there was no effect on the growth of subcutaneous MDA-MB-231 tumors, with a 10-fold lower HER2 density (Figs. 4A and 4C). The selected NOAEL was based on the absence of hematopoietic toxicity at 2 wk after intraperitoneal administration of 111In-NLS-trastuzumab to non–tumor-bearing normal BALB/c mice. Although the difference in the actual tumor volumes at the end of the 60-d observation period was small, 111In-NLS-trastuzumab was more effective at controlling tumor growth than was 111In-trastuzumab without NLS modification or an equivalent mass dose amount of unlabeled trastuzumab (Fig. 4A). 111In-NLS-trastuzumab administered as 2 doses at the NOAEL separated by 2 wk was also more effective than equivalent mass doses of unlabeled trastuzumab at prolonging the survival of mice with MDA-MB-361 tumors (Fig. 5). These results suggest that this radioimmunotherapeutic agent could be more effective than trastuzumab for treatment of HER2-overexpressing breast cancer tumors in humans.
Mattes and Goldenberg (16) previously examined the antitumor effects and toxicity of 111In-labeled monoclonal antibody 4D5, the murine analog of trastuzumab, in athymic mice with subcutaneous HER2-positive SKOV-3 ovarian cancer xenografts. In these mice receiving the maximum tolerated dose (2.7 MBq/g of body weight, equivalent to about 54 MBq for a 20-g mouse), compared with control mice receiving irrelevant 111In-labeled antibodies or no treatment, there was a 3.6-fold increase in the median time for tumors to reach 0.5 cm3. These doses of 111In-4D5 were about 6-fold higher than the single doses of 111In-NLS-trastuzumab (9.25 MBq) found effective for controlling the growth of MDA-MB-361 tumors. It is difficult to compare studies using different tumor xenograft models and antibodies, but the HER2 density on SKOV-3 cells is higher than that on MDA-MB-361 cells (1 × 106 vs. 5 × 105 receptors per cell) (17). Furthermore, the tumor uptake at 3 d after injection of 111In-4D5 (mixed with 111In-21.1, a second HER2 antibody to enhance tumor localization) in the SKOV-3 model was higher than that reported by us for 111In-NLS-trastuzumab in mice with MDA-MB-361 tumors (21 vs. 12 percentage injected dose per gram [%ID/g], respectively) (9). These comparisons point to an increased effectiveness of 111In-NLS-trastuzumab, compared with 111In-labeled HER2 antibodies without NLS peptide modifications, for inhibiting the growth of HER2-positive tumors in vivo. This increased effectiveness was observed in our study, which revealed that 111In-NLS-trastuzumab was more effective than 111In-trastuzumab for inhibiting the growth of MDA-MB-361 tumors (Fig. 4A). The higher potency of 111In-NLS-trastuzumab is likely to be due to greater DNA damage caused by the close proximity of the Auger electron emissions—such proximal emissions that are due to nuclear importation mediated by the interaction of NLS with importins α/β (8). Previously we found that 111In-NLS-trastuzumab exhibited 2- to 3-fold greater nuclear uptake in MDA-MB-361 tumors in vivo than did 111In-trastuzumab (9). Nuclear localization also significantly increased the cytotoxicity in vitro of 111In-NLS-trastuzumab, compared with 111In-trastuzumab, on SKBR-3 and MDA-MB-361 breast cancer cells in clonogenic assays (9).
Toxicity toward normal tissues is dose-limiting for radioimmunotherapy with 131I- or 90Y-radiolabeled antibodies because of nonspecific irradiation and killing of hematopoietic stem cells through a cross-fire effect from the moderate- to high-energy and long-range (2–10 mm) β-particles (11). Auger electron emitters do not have a cross-fire effect and in most cases require internalization and nuclear importation to be lethal to cells. A limitation to our study, however, is that trastuzumab does not recognize the murine c-erbB2 homolog of HER2, and thus it was not possible to evaluate HER2-mediated normal-tissue toxicities from 111In-NLS-trastuzumab in the tumor xenograft model used (18). Transgenic mice that express the human HER2 gene under the whey acidic protein promoter may be useful for assessing these toxicities from 111In-NLS-trastuzumab (19). Nonetheless, there were no nonspecific toxicities toward the hematopoietic system at 2 wk after a single dose (9.25 MBq; 4 mg/kg) of 111In-NLS-trastuzumab; higher doses decreased WBC, platelet, and RBC counts and hemoglobin concentrations (Fig. 1). In humans, hematopoietic stem cells have minimal HER2 expression and are not expected to be targeted and killed by 111In-NLS-trastuzumab (12,20). Nonspecific myelotoxicity may occur from the γ-emissions of 111In, particularly when the radiopharmaceutical is administered at high doses. These non–HER2-mediated effects on the hematopoietic system were observed for 111In-NLS-trastuzumab (Fig. 1) and 111In-NLS-hIgG. These results are consistent with the nonspecific toxicity previously observed by Govindan et al. (21) for CD74-positive Raji lymphoma cells treated with high concentrations of 111In-labeled irrelevant antibodies (possibly due to the γ-emissions), whereas much lower concentrations of 111In-LL1 CD74 antibodies were specifically toxic to the cells. The nonspecific hematopoietic toxicity of 111In-NLS-trastuzumab was greater than that found for 111In-labeled human epidermal growth factor (111In-hEGF), an Auger electron–emitting radiotherapeutic agent for epidermal growth factor receptor–overexpressing breast cancer; this difference is likely explained by the much longer residence time of 111In-NLS-trastuzumab in the blood, compared with 111In-hEGF (elimination half-life, 33.7 h vs. 24–36 min, respectively; Table 1), which permits greater nonspecific irradiation of hematopoietic stem cells from circulating radioactivity (22).
No hepatic or renal toxicity was noted for 111In-NLS-trastuzumab, despite the accumulation of high concentrations of radioactivity in these tissues (11–12 %ID/g and 7–8 %ID/g, respectively) (9). These results are consistent with those for 111In-hEGF, in which no hepatic or renal toxicity was observed in BALB/c mice or New Zealand White rabbits administered high doses of this radiopharmaceutical (22). It has recently been suggested that heterogeneities in absorbed dose deposition in radiation-sensitive glomeruli can explain the lack of renal toxicity from high doses of 111In-pentetreotide, compared with 177Lu- or 90Y-DOTATOC (which are toxic to the kidneys at much lower doses unless renal protection is provided) (23). Such microdosimetry considerations may similarly help to explain the absence of toxicity from 111In-NLS-trastuzumab or 111In-hEGF to the liver or kidneys. HER2-mediated cardiotoxicity from 111In-NLS-trastuzumab in humans is possible because trastuzumab has been associated with an increased risk for cardiac dysfunction, especially when combined with anthracyclines (24). However, HER2 receptors have been found at minimal levels in the myocardium of breast cancer patients (20), and it has been argued that the cardiotoxicity of trastuzumab–anthracycline regimens may be related to anthracycline-induced myocardial injury rather than to trastuzumab (25,26). No non–HER2-mediated cardiotoxicity was noted in our study.
The NOAEL dose of 111In-NLS-trastuzumab (9.25 MBq; 4 mg/kg) selected for treatment was conservative. Mattes and Goldenberg (16) safely administered radioactivity doses of 111In-4D5 as high as 59.2–66.6 MBq to non–tumor-bearing mice. Behr et al. (27) administered doses of 111In-labeled CO17-1A antibodies up to 85.1 MBq to athymic mice without lethality; even higher doses up to 118.4 MBq were safely administered, with bone marrow stem cell support. Thus, it may be feasible to administer higher doses of 111In-NLS-trastuzumab than the NOAEL examined in this study to achieve even greater antitumor effects. Another potential strategy for enhancing the antitumor effects of 111In-NLS-trastuzumab may be to combine this radiopharmaceutical with a radiosensitizer. We recently reported that the cytotoxicity of 111In-NLS-trastuzumab in vitro toward MDA-MB-361 cells in clonogenic assays was enhanced 1.4- to 1.7-fold when combined with low concentrations of methotrexate intended to deplete the nucleotide pool required for DNA repair (10).
CONCLUSION
Single doses of 111In-NLS-trastuzumab at the NOAEL (9.25 MBq; 4 mg/kg) had potent and selective tumor growth–inhibitory effects in vivo against subcutaneous HER2-overexpressing MDA-MB-361 human breast cancer xenografts in athymic mice, and 2 doses separated by 2 wk prolonged the survival of tumor-bearing mice. At the NOAEL, this radiopharmaceutical was well tolerated, with no decreases noted in WBC, RBC, or platelet counts or hemoglobin concentrations or increases in serum ALT or creatinine levels. On the basis of these results, 111In-NLS-trastuzmab is a highly promising targeted radioimmunotherapeutic agent for HER2-overexpressing tumors in humans.
Acknowledgments
This research was supported by grants 016456 and 019513 from the Canadian Breast Cancer Research Alliance, with funds from the Canadian Cancer Society, by a doctoral fellowship to Danny Costantini from the Canadian Breast Cancer Foundation (Ontario Chapter), and by a Canadian Institute of Health Research Frederick Banting Doctoral Scholarship to Kristin McLarty.
Footnotes
-
COPYRIGHT © 2010 by the Society of Nuclear Medicine, Inc.
References
- Received for publication November 3, 2009.
- Accepted for publication March 16, 2010.