Abstract
Since its introduction in 1998, dual-modality PET/CT imaging has received great attention in the medical community. For the first time, patients can be examined with both CT and PET in a single examination. A whole-body survey is the standard mode of acquisition. The CT images are used for anatomic reference of the tracer uptake patterns imaged in PET, as well as for attenuation correction of the PET data. The routine use of CT-based attenuation correction and user preferences for the quality and type of the CT examination have led to the introduction of different PET/CT scanning protocols. Discussion: Two general approaches to PET/CT imaging can be distinguished today. One uses CT as a fast transmission source with little additional information for anatomic labeling. The other uses CT as a fast transmission source as well as a state-of-the-art diagnostic tool to maximize image quality using optimal acquisition parameters together with oral and intravenous contrast agents. Variations of these approaches share common concerns about image artifacts that result from mismatches in respiration and patient positioning between the CT and the PET examinations. Protocol requirements for the more complex radiologic PET/CT scenario also include alternative contrast application schemes or modifications to the attenuation correction procedure to handle CT contrast agents appropriately. Conclusion: High-quality PET/CT studies can be provided routinely with existing PET/CT technology that is used efficiently by trained and motivated technologists and physicians. Only then will the potential diagnostic benefit of this new imaging modality be explored fully.
It has been hypothesized for some time now that complementary functional and anatomic imaging, such as that performed with PET and CT, allows for improved diagnosis and thus better patient care in clinical oncology (1,2). Software-based algorithms to align independently acquired PET and CT image sets have been available for several years, and an alternative hardware approach has been introduced only recently (3,4). Based on a proof-of-principle prototype, various PET/CT system designs have been developed and are available today (5). Several advantages are associated with combined PET/CT imaging compared with retrospective or prospective software-based approaches to align complementary image data. Most important, the patient undergoing a combined PET/CT examinations is not moved physically (except for the translation of the bed) between CT and PET acquisition, thus limiting misalignment from repositioning. In combined PET/CT, the lengthy standard PET transmission scan is no longer needed, because transmission data from the CT acquisition of the combined PET/CT examinations can be used for attenuation (and scatter) correction of the complementary emission data. This results in greatly reduced total examination times and in potentially reduced costs when standard PET transmission sources are not installed in the combined tomograph. Patients further benefit from the logistic advantages of being referred for only one examination rather than two separate scans on 2 days. In addition, a joint report on the combined PET/CT data, in theory, can investigate all findings with the expertise of a radiologist and a nuclear medicine physician.
STANDARD FDG PET/CT IMAGING PROTOCOL
The main strength of PET imaging in clinical oncology is to detect primary and metastatic disease by means of a whole-body survey with a single injection of a given amount of a radiotracer, such as 2-18F-FDG. In contrast, CT imaging has been used most frequently to image the anatomy of only a single organ (e.g., liver), or to review examination ranges of only limited axial extent (e.g., thorax, neck). With the introduction of multislice CT technology (6) and optimized exposure management, this concept of locoregional examinations has been changed, and extended in vivo surveys of patient anatomy have become more popular. The ability to acquire anatomic information over extended imaging ranges at reasonable patient exposure levels (6) underlies the main concept of combined PET/CT imaging, which is to supplement metabolic information from a whole-body PET study with detailed information on the corresponding anatomy of the patient for improved diagnostic accuracy. Therefore, most, if not all, PET/CT imaging protocols are based on standard whole-body (7) PET acquisition protocols, which involve a pre- or postinjection transmission scan followed by an emission scan covering the same axial imaging range.
Standard 18F-FDG imaging protocols with PET/CT can be divided into 7 steps (Fig. 1). In contrast to a stand-alone PET, a topogram (overview scan) is acquired for accurate definition of the axial examination range. Because of the essentially nonexisting contamination from the emission activity of the patient, CT transmission data are always collected in postinjection mode. Depending on the anticipated use of the combined PET/CT data (i.e., initial diagnosis or follow-up), additional requirements may be imposed on the acquisition protocol, such as the use of breath-hold commands or the administration of CT contrast agents. In general, however, a PET/CT protocol is similar to a standard PET protocol, with the exception that the standard PET transmission data are replaced by the CT transmission data. Table 1 compares important requirements for each of the acquisition steps in combined PET/CT and separate CT and PET.
Standard FDG-PET/CT imaging protocol. The patient is positioned on a common patient handling system in front of the combined gantry. First, a topogram is used to define the co-axial imaging range (orange). The spiral CT scan (gray box) precedes the emission scan (green box). CT images are reconstructed on-line and used for the purpose of automatic attenuation correction of the corresponding emission data (blue box). Black and blue arrows indicate acquisition and data processing streams, respectively.
Acquisition Protocol Considerations in PET, CT, and PET/CT Whole-Body Imaging
Patient Preparation
Proper patient preparation for whole-body PET studies has been described in detail, for example by Hamblen and Lowe (8). These criteria apply also to patient preparation for PET/CT studies. Patients should be questioned independently for allergies to iodine-based CT contrast agents if these are to be administered intravenously during the course of a PET/CT study. Oral contrast agents, on the other hand, typically do not require special premedication or testing. Depending on whether and which oral CT contrast agents are given, patients are asked to drink up to 1,500 mL of oral contrast solution during the 18F-FDG uptake phase. The injected dose of 18F-FDG may vary between 300 and 700 MBq, depending on scanner characteristics, such as PET detector material and acquisition mode.
Patient Positioning
Before the examination, patients should remove all metal (e.g., bracelets, dental braces, pants with zippers, etc.) that could lead to streak artifacts on the CT transmission scan. Patients must be positioned comfortably on the examination pallet. Several PET/CT tomographs offer a uniform 70-cm gantry opening, larger than the 60-cm standard PET opening. Patients can be positioned in the larger opening with their arms raised above their heads, which is standard practice in CT. Patients should be supported with adequate positioning aids (e.g., knee, head and neck, and arm supports) to limit involuntary motion that may lead to general or local misalignment during the combined examinations.
Overview Scan
PET/CT examinations start with the acquisition of a topogram or scout scan that is an x-ray image overview scan of the patient. The topogram is acquired during continuous table motion, with the x-ray tube/detector assembly typically locked in either frontal or lateral position (or any position in between), thus generating an anatomic overview image that is similar to a conventional x-ray at a given projection. The topogram is used to define the axial examination range of the PET/CT study. The axial extent of the CT and PET portions of the combined examinations are thereby matched to ensure fully quantitative attenuation and scatter correction of the emission data. Visual markers for the measured transverse field of view of the CT (50 cm) and the PET (60 cm) are displayed on the topogram. These markers guide the technologist to ensure that all body parts are positioned inside the smaller transverse field of view of the CT. Patients should be repositioned before the CT scan when truncation of the anatomy is predicted by the topogram.
CT Acquisition
After the definition of the coaxial imaging range, the patient is moved automatically into the CT field of view for the transmission scan. Most PET/CT users acquire a single continuous spiral CT scan. The start of the CT scan is delayed by 20–50 s if intravenous contrasts are administered according to standard CT protocols. A breath-hold command can be given to match the anatomy more closely to the physiology of the patient, which is acquired in the free-breathing emission scan.
PET Acquisition
After completion of the CT scan, the patient is advanced to the field of view of the PET, to the rear of the combined gantry, where emission scanning commences in the caudocranial direction, starting at the thighs to limit artifacts from the FDG metabolite excretion into the urinary system. Depending on the axial co-scan range and the emission time allotted for an individual bed position, the combined scanning is completed in 30 min (9) or less (10).
Data Processing and Reconstruction
CT image reconstruction is in parallel with emission acquisition. Because the reconstruction of a single CT image takes <1 s, the CT images are ready for attenuation correction processing before the first emission scan is completed. The calculation of the attenuation correction factors (ACF) from the CT transmission data (Fig. 1) is based on the algorithm outlined by Kinahan et al. (11).
Emission images are reconstructed consecutively with the completion of the emission acquisition and using the available ACFs. Almost immediately after the completion of the last bed position of the PET scan, all emission images are available and assembled into a whole-body volume.
Image Analysis and Reporting
Depending on the reviewer and the objective of the study (Table 2), any or all of the following image sets are needed: CT, corrected PET, and noncorrected PET. In addition, several postacquisition reconstruction tasks may be required to create CT image sets with alternate filter and window-level settings (e.g., lung-window). Special viewing and fusion tools typically are available with standard PET/CT software to allow scrolling through any of the selected individual and fused image volumes as well as side-by-side viewing.
Objectives and Requirements of Clinical PET/CT Imaging
OPTIMIZATION OF STANDARD 18F-FDG PET/CT IMAGING PROTOCOLS
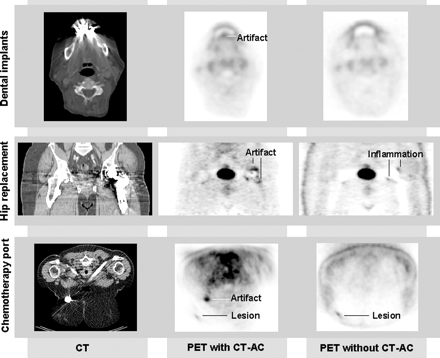
With the routine availability of CT transmission images and CT-based attenuation correction, some pitfalls may arise for PET/CT users with only limited PET or CT experience. Figure 2 illustrates some concerns in combined PET/CT imaging about the use of CT images instead of standard PET transmission images. Most of these concerns relate to potential artifacts in PET/CT images that result from the nature of the interaction of low-energy x-ray photons with tissues and the subsequent propagation of CT image artifacts into attenuation-corrected PET images. In addition, a number of PET/CT users believe that acquiring the CT with standard dose protocols is inadequate in combined PET/CT imaging and that, therefore, PET/CT protocols should use reduced (if not minimized) dose settings for routine imaging (12,13). Therefore, combined PET/CT imaging requires addressing the often conflicting goals for PET and CT.
Optimization tasks of PET/CT imaging protocols relate to clinical and methodological concerns: What CT dose levels are adequate for diagnostic purposes and CT attenuation correction? Should CT contrast agents be used? Should CT standards be adopted for the position of the arms, respiration protocols, or for the administration of CT contrast agents? Will metal implants introduce artifacts into corrected PET images?
Today, with about 5 years of clinical PET/CT imaging experience, comparatively little data are available on the true impact of PET/CT imaging on diagnostic accuracy and patient management (2,12,14). The shortage of clinical data and the lack of optimal protocol parameters naturally contribute to a dispute over the utilization of PET/CT imaging for oncology patient work-up (15). Both clinical and scientific factors dictate the requirements for performing PET/CT. In practice, two general approaches can be distinguished (Table 2).
In the first, a state-of-the-art diagnostic CT is not needed or indicated, because the patient had previously undergone a complete CT examination. The CT, as part of the PET/CT, is used instead as a fast transmission measurement for attenuation correction and for anatomic labeling of PET findings.
In the second approach, a state-of-the-art, diagnostic CT is clinically indicated. Typically, the CT, as part of the PET/CT, is acquired using oral or intravenous contrast agents to maximize the diagnostic information on anatomy and tumor vascularization. In addition, the CT is used as a fast transmission source for attenuation correction, anatomic labeling, or referencing of the PET results.
In the first approach, the PET/CT is performed in addition to an independent diagnostic CT examination, whereas, in the alternative approach, the PET/CT replaces a clinical CT and a clinical PET. A third approach to PET/CT imaging would be to accept the CT portion of the combined PET/CT as a very fast transmission scan option only, without exploring any anatomic labeling of the corresponding PET findings. This concept of PET/CT, however, is merely a fast PET imaging procedure and will not be considered here.
In both scenarios (Table 2) and in contrast to standard PET procedures, emission data are corrected routinely for attenuation by using the available CT transmission information. Current PET/CT technology (16) addresses most of the requirements of each scenario, which are very similar in technical nature (Fig. 1) but differ on requirements for the quality of the CT acquisition. In practice, clinical needs, site preferences, and even legal concerns also affect these requirements. To improve overall PET/CT image quality in clinical routine and to address the requirements of diagnostic PET/CT imaging, several advances have been made in the design of PET/CT acquisition protocols and in the adoption of alternative CT contrast and respiration techniques.
GENERAL ASPECTS OF 18F-FDG PET/CT IMAGING PROTOCOLS
Patient Positioning
With attenuation correction based on the use of CT instead of 68Ge-rod sources, whole-body scan times are reduced by as much as a third (9) to 30 min or less (10,16). From our experience with about 3,500 PET/CT studies, most patients tolerate being scanned with arms raised and supported above the head. To facilitate comfortable positioning of the arms, several low-attenuation CT positioning devices are available and can be adapted for PET/CT imaging. By raising the arms for the duration of the whole-body scan and leaving them outside the measured CT field of view, scatter artifacts in the body are much reduced (Fig. 3) and counting statistics of the corresponding emission scan are increased. Conversely, for head-and-neck investigations, the area is scanned with arms down.
(A) Topogram scans of 3 patients with different arm positions. Selected transverse CT images (center 50 Hounsfield units [HU], width 300 HU) at level of midliver are shown to illustrate magnitude of streaking artifacts. Streaks are reduced most in case when both arms are raised (right). (B) Male patient with lymphoma. Fused image shows involvement of retrocrural lymph node (arrow). Streak artifacts originating from positioning of patient with arms down degrade quality of CT and fused PET/CT images.
Independent of the coaxial imaging range, all patients should be supported with a proper knee rest for the duration of the combined scan (Fig. 4). When using foam pallets or vacuum bags, no artifacts are typically introduced into the CT transmission data or, subsequently, into the corrected emission data. When using custom-made positioning aids, however, phantom studies should be performed first to validate artifact-free positioning. This is particularly important for radiation therapy positioning set ups.
Patient positioning with arm rest (A) and knee rest (B) for whole-body PET/CT studies. Arm and knee rests are made of low-density foam with CT attenuation close to that of air. No artifacts are generated from these positioning aids, as is illustrated in transverse CT images to right (window levels center, C, and width, W, in Hounsfield units).
Truncation Artifacts
Careful patient positioning supported by positioning aids is important also for reducing truncation artifacts in combined PET/CT imaging. Spiral CT technology today offers a measured transverse field of view of 50 cm, which is 10 cm less than the transverse PET imaging field. Therefore, truncation artifacts are frequently observed in combined PET/CT imaging of very large patients or patients imaged with arms down (17) (Fig. 5). If the patient extends beyond the boundaries of the transverse CT field of view, part of the anatomy is not reconstructed in CT. It will not be available to the CT-based attenuation correction procedure, which is based on fully reconstructed CT images. Thus, if truncation exists and is not corrected for, the reconstructed emission images appear to be masked by the truncated CT (18,19). The tracer distribution is then only partially recovered outside the measured CT field of view (Fig. 5B), and some bias of the reconstructed activity distribution inside the common field of view is observed.
Difference (A) in maximum transverse field of view of CT (blue) and PET (orange) may lead to truncation artifacts in PET/CT images (B). CT images of large patients or patients with arms down appear truncated at sides, and derived attenuation maps appear to mask corrected emission activity. (B) CT, uncorrected, and corrected PET. (C) Truncated attenuation information may be recovered before CT-based attenuation correction.
Several algorithms have been suggested to extend the truncated CT projections and to recover truncated parts of the measured attenuation map (17,18). If applied to CT images before CT-based attenuation correction, these correction algorithms will help to recover the tracer distributions measured with the complementary emission data. Additional work is needed, however, to make such algorithms available routinely for clinical diagnostics.
Respiration Artifacts
Mismatches of breathing patterns in combined PET/CT examinations have been described as a source of potential artifacts in emission images after CT-based attenuation correction (20). These artifacts are particularly severe when standard breath-hold techniques (e.g., scanning at maximum inspiration) are transferred directly from clinical CT to combined PET/CT without further adaptation (Fig. 6).
(A) Significant respiration mismatch between CT and PET results in severe artifacts in corrected emission images. (B) Free breathing during spiral CT scan may cause artifacts that propagate into corrected PET, which is acquired in free breathing over many respiration cycles. (C) Using special breathing instructions, respiration-induced artifacts and mismatches may be reduced in majority of cases.
The areas most affected by respiration are the lower thorax, anterior chest wall, and liver (21). In the absence of routinely available respiratory gating options, the anatomy of the patient captured during the CT scan must be matched to the PET images that are acquired over the course of multiple breathing cycles. Goerres et al. (22) have compared the quality of PET/CT image alignment in the thorax and abdomen for breath-hold and normal breathing during the CT portion of the combined acquisition. They found normal expiration and free breathing to provide the best match in the thorax in 53% and 27% of patients, respectively (22). CT and PET alignment accuracy for abdominal structures was similarly satisfactory when acquiring the CT either in free breathing or in normal expiration (23).
The applicability of the normal expiration protocol, however, is limited to PET/CT tomographs with very fast CT components or CT protocols that use large table feeds per rotation. Acquiring the CT in normal expiration over the entire imaging range may not be feasible when scanning uncooperative or very sick patients. Therefore, an alternative, limited breath-hold protocol has been suggested (20). Patients are asked to hold their breath in normal expiration only for the time that the CT takes to cover the lower lung and liver, which is typically <15 s. Instructing the patient before the PET/CT examinations on the breath-hold command is essential in avoiding serious respiration artifacts. When respiration commands are not tolerated by the patient or when respiration-induced misalignment persists and appears to introduce artifacts into the corrected PET, it is advisable to also reconstruct the emission data without attenuation correction and to carefully review the two sets of fused PET/CT images.
CT Contrast Agents
The utility and use of CT contrast agents in PET/CT imaging are still the subjects of some dispute when defining clinical acquisition protocols (24). In our opinion, the question of whether to use CT contrast agents for PET/CT studies arises only in the state-of-the art diagnostic CT imaging scenario (Table 2) that requires state-of-the-art CT scans (25). In addition to the clinical impact, the point of concern is whether or not CT contrast agents can be administered at no expense to the combined image quality when only CT-based attenuation correction is available (Fig. 2). On occasion, focally increased concentrations of high-density intravenous or oral contrast agents are seen on CT, which results in artificial tracer uptake patterns on the corrected PET images of PET/CT studies. These observations are further discussed by Antoch et al. (24) elsewhere in this Supplement.
In the absence of reliable and automated correction algorithms for CT contrast, alternative contrast administration protocols (Fig. 7) have been proposed for PET/CT imaging (24). For example, high-density focal artifacts from intravenous bolus injection may be avoided by applying the same contrast volume with an adaptive pressure pump or, alternatively, a saline chaser (26) after the intravenous contrast agent has been injected. In addition, the use of negative oral contrast agents for the enhancement of the gastrointestinal tract has been proposed for PET/CT imaging (Fig. 7B) to avoid overestimation of attenuation coefficients from high-Z materials (27).
Alternative schemes of application of intravenous contrasts (A) and negative oral contrast agents (B) are being pursued to avoid contrast-induced artifacts on PET/CT images while providing acceptable image quality for radiologists.
Metal Implants
Many oncology patients have artificial metal implants, such as chemotherapy ports, metal braces in the spinal region, artificial joints, or dental fillings. In standard PET transmission scanning, metal implants cause little or no artifacts. However, these artifacts can be severe at CT energies (Fig. 8), because of the significantly higher photon absorption from high-Z materials (e.g., metals) compared with low-Z materials (e.g., soft tissues) at CT energies. In standard CT, iterative algorithms are available to correct for beam hardening and scatter from metal implants, but these corrective steps have not yet been implemented routinely in PET/CT protocols.
High-density implants generate streak artifacts on CT, which may translate into distortions and biases of recovered tracer activity. PET images without CT-based attenuation correction (CT-AC) may help to interpret metal-induced artifacts.
Several PET/CT users, therefore, have reported on the effect of metal artifacts on image quality in PET/CT studies of the head and neck and around hip replacements (28–30). Another important aspect when scanning oncology patients is the generation of focal artificial uptake patterns of 18F-FDG from chemotherapy ports. Because of the high-density properties of these ports (titanium) and the low-attenuation properties of the surrounding tissues, artificial 18F-FDG foci are likely to be generated. These foci may be misleading in the diagnosis, particularly when true lesions are present in the vicinity of the port (Fig. 8C). It is therefore advisable to also reconstruct emission images without attenuation correction whenever metal implants are present (28–30).
Combined Scanning and Joint Report
Current software packages allow the correlated review of PET and CT data either side by side or in fused mode. Some image-review stations also allow extensive quantitative analysis of the combined data as part of the clinical reporting. State-of-the-art reporting tools, however, lack adequate capabilities to display and review serial PET/CT studies of patients in therapy follow-up.
The primary concept of PET/CT is the immediate transformation of the functional information from PET into the spatial coordinate system of CT. The second and no less attractive concept is to identify any relevant PET findings with normal or pathologic morphologic background. Similar to the different perspectives on the operation of a PET/CT system (Table 2), different opinions exist for the transformation of the main findings of PET/CT imaging into a clinical report through the image review process (31). Obviously, a joint report is necessary and is an easy task when CT and PET findings are concordant. However, there is no accepted rule for dealing with contradictory PET and CT findings. A consensus finding could be facilitated by the joint efforts of the evaluating physicians of complementary radiology and nuclear medicine expertise, or, in settings in which this expertise is provided by the same physician, by getting the expert opinion of subspecialized medical professionals (e.g., a head-and-neck surgeon). Finally, joint reports not only benefit from the clinical expertise of both a radiologist and a nuclear medicine specialist but also discern potential image artifacts and help to eliminate image artifacts a priori in subsequent imaging.
SPECIAL FDG PET/CT IMAGING PROTOCOLS
When combining the diagnostic power of CT and PET imaging, a straightforward application of standard whole-body imaging protocols may not yield the best image quality for all indications. For example, PET/CT studies involving the head and neck frequently suffer from local misalignment in the region of the neck (30). The cause of this local misalignment (Fig. 9A) is the relaxation of neck muscles, with resulting movement of the neck within the 20-min time delay between CT and PET acquisitions in a standard whole-body protocol.
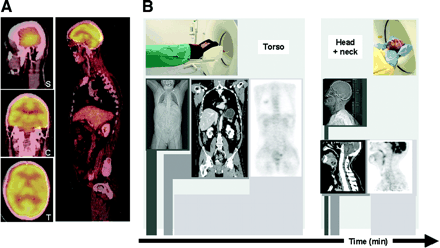
(A) Without efficient positioning aids, misalignment from muscle relaxation and involuntary patient motion may be observed in head and neck. (B) Whole-body imaging ranges can be separated into neck and thorax scan ranges, each with its own optimized positioning, acquisition, and reconstruction parameters for improved imaging at little cost in examination time.
To avoid patient motion in the neck, where accurate alignment of CT and PET information is critical, and to optimize imaging parameters, we have introduced a linked neck/torso imaging protocol with slightly overlapping coaxial imaging ranges from the neck and torso (Fig. 9B). First, a PET/CT study of the torso is performed with the patient positioned with arms up, as described in the standard whole-body protocol. Assuming a 5-bed–position emission scan with a scan time of 3.5 min per bed, total examination time including the topogram is <20 min. The patient is then asked to lower the arms and keep them close to the trunk for the dedicated head-and-neck scan. A vacuum pillow is used to support the neck and head for the duration of the second examination. The PET/CT study of the neck is performed for two bed positions at 6 min per step. Total examination time for the torso and neck coverage, including a 2-min period to reposition the patient between scans, is thus about 35 min, still significantly shorter than an equivalent PET study. The administration of intravenous contrast agents is adjusted according to the two co-axial imaging ranges covering the thorax and the neck, respectively (Table 3).
Diagnostic PET/CT Acquisition Parameters for Whole-Body and Combined Head/Neck–Torso Protocol
There are several advantages to this protocol. First, the imaging parameters can be adjusted to torso (i.e., lung) and neck scanning (Table 3). This is important when utilizing contrast agents and making use of the range of available CT scan parameters. Second, the patient can be positioned and supported more efficiently for each scan. Third, the patient can get off the table between the two partial examinations if needed. While we thus improve overall image quality, the total examination time is only slightly increased.
We routinely complement the standard whole-body PET/CT examination in patients with breast cancer with a second, locoregional scan of the breast, with the patient in prone position and the breasts in a hanging configuration, which is standard practice in CT examinations of the breast (32). Prone positioning was also shown to be more effective than supine imaging for the diagnosis of breast cancer with 18F-FDG PET (33). We perform the dedicated breast PET/CT examination without the administration of intravenous contrast and reduce the CT exposure to 24 mAs. In our experience, scanning the hanging breast facilitates a better separation of breast tissue from the chest wall.
In our hospital, combined PET/CT imaging is part of pre- and posttherapy management of patients scheduled for radiation therapy of head and neck cancer and lung cancer. These patients follow the neck/torso acquisition protocol outline described previously (Fig. 9). Care is taken when positioning these patients (with masks on) on the patient support platform of the PET/CT to approximate as closely as possible the radiation treatment planning and treatment position. Upon completion and review of the scan, PET and CT Digital Imaging and Communications in Medicine (DICOM) images are transferred directly to the radiation treatment planning software, where the combined imaging information is entered into the treatment planning process.
DISCUSSION
After several years of clinical PET/CT experience, an important conclusion is that diagnostic PET/CT protocols require a greater amount of attention to the preparation of the patient and operation of the tomograph than, perhaps, for either imaging modality alone. For example, PET/CT users without previous CT experience have quickly learned to appreciate the sensitivity of CT image quality to patient motion and high-density implants, and radiology-trained users have become familiar with extended examination times. In light of the methodologic challenges of combined PET/CT imaging (Fig. 2), which include the intrinsic mismatch of respiration in CT and PET and the routine use of CT contrast agents, several users prefer to operate the PET/CT with substandard CT acquisition parameters in addition to separate clinical CT scan (Table 2). However, the increased radiation burden to the patient from repeated CT examinations should be considered. Furthermore, scientific evidence is still needed to demonstrate that PET/CT without CT contrast is equivalent to or better than CT alone with contrast agents.
The use of CT contrast agents in PET/CT imaging is no longer an obstacle to clinical PET/CT imaging. The methods of CT-based attenuation correction are well understood, and several modifications to the inherent scaling models were suggested to account for the presence of high-density contrast agents on CT images used for attenuation correction, for example (19,20). Meanwhile, initial clinical experiences with alternative contrast injection schemes and negative oral contrast agents (24) have shown good results and may serve as more practical methods of choice in routine PET/CT imaging before more sophisticated yet robust correction algorithms become available.
Despite advances in reducing the spatial mismatch between CT and PET data and in lowering potential biases in the reconstructed tracer distribution, the subject of optimum CT dose parameters is still in great dispute. Typically, the effective dose in clinical CT is on the order of 3–15 mSv (35), which is about the same (9 mSv) for an average PET study with 370 MBq 18F-FDG (36). Several technical features are available to manage patient exposure in a variety of CT imaging scenarios. Some CT systems offer x-ray tube-current modulation, so that the photon flux is increased somewhat in the lateral views but reduced significantly in the anterior and posterior direction. Tube-current modulations have been shown to reduce effective patient exposure by as much as 20% in adults (37) and 23% in children (38). Kalra et al. reported on a 50% reduction in x-ray tube current without a degradation of clinical image quality in abdominal CT of normal-weight patients up to 90 kg (39).
If a state-of-the art diagnostic CT is not mandated by the clinician in PET/CT imaging, some users seek additional reduction of the patient dose from CT (12–14), albeit often at the expense of diagnostic image quality of the CT. If lower-quality CT images are acceptable, exposure levels can be reduced dramatically, but little supportive data is available that demonstrate equivalent diagnostic accuracy of low- and high-dose CT in combination with PET (12–14).
Together with technical improvements in both CT and PET technology (16), several updated combined tomograph designs have been introduced recently. These designs aim primarily at increased PET performance, such as higher sensitivities and faster scan times with fast detector materials and improved detector electronics, which preferentially benefit users who administer larger doses of 18F-FDG. By introducing 16-row CT technology into combined PET/CT designs, the advantages of very fast and high-resolution volume coverage by CT are translated directly into the context of combined anatomic and functional imaging. Durable CT rotation speeds of 0.5 s and less help to reduce respiration-induced artifacts and to image the anatomy of multiple organs at peak enhancement after intravenous contrast injection. Alternatively, combined imaging protocols that include multiple, contiguous spiral CT scans to cover the extended axial imaging range for different peak enhancements of a single intravenous contrast injection now appear feasible.
With ever-increasing data volumes, however, there is a downside to existing PET/CT imaging scenarios. In a recent study (40), the logistic benefits and necessary image review times of multislice CT were compared with those of single-slice CT. The authors concluded that although the actual clinical CT examination was finished in less time with a multi-row spiral CT, the increased number of images and information could easily lead to longer review times unless more efficient visualization tools become available. PET/CT is a complex imaging technique that generates more information than either imaging modality alone. It also appears to be a promising tool for monitoring therapy response. Therefore, novel, and probably automated software tools must be developed that allow the registration and review of serial PET/CT image sets and the potential incorporation of additional diagnostic studies, such as MRI. It is then possible that in the not-too-distant future a PET/CT examination will take less time than the actual image review process.
We already need a higher flexibility of PET/CT acquisition protocols in the search for alternative imaging protocols. For example, the entire coaxial imaging range could be covered by a combination of a low-dose and a shorter diagnostic CT scan, or patients could be scanned in emission mode only before a decision is made on the need and extent of a complementary CT transmission scan. With more variable acquisition schemes at hand and with increasing levels of experience and expertise, PET/CT users may then extract the most useful indications for PET/CT imaging. Being able to choose between various acquisition parameters and to set up optimized protocols will lead to a diversification of PET/CT imaging techniques. The optimization of PET/CT protocols, however, will always be driven to a great extent by the needs and preferences of the users.
CONCLUSION
Since the inception of combined PET/CT imaging in clinical oncology a few years ago, several different imaging approaches and protocols have been proposed. Independent of the preferred imaging scenario, careful image review and consideration of potential artifacts arising from the routine use of CT-based attenuation correction are advised in clinical PET/CT imaging. Nevertheless, with growing experience and with technologic improvements of the existing PET/CT hardware, high-quality PET/CT studies can be provided routinely in clinical practice. By using this new technology efficiently in the hands of trained and motivated technologists and physicians, its potential diagnostic benefit can be explored fully.
Acknowledgments
We would like to thank our technologists Bärbel Terschüren, RT; Lydia Schostok, RT; Dorothea Prosch-Plotek, RT; Sandra Pabst, RT; Gianina Markese, RT; Sandra Heistrüvers, RT; and Slavko Maric, RT, for their encouragement in providing the best patient care to our PET/CT patients. We appreciate the support of Simone Marnitz, MD, Sarah Grehl, MD, and Andrea Flühs in preparation of our radiation treatment planning imaging protocols. We gratefully acknowledge the assistance of CPS Innovations, Inc., in making available to us several special patient positioning aids.
Footnotes
Received Sep. 25, 2003; revision accepted Nov. 7, 2003.
For correspondence or reprints contact: Thomas Beyer, PhD, Department of Nuclear Medicine, University Hospital Essen, Hufelandstr 55, 45122 Essen, Germany.
E-mail: thomas.beyer{at}uni-essen.de