Abstract
Tumor targeting and therapeutic efficacy of 177Lu-labeled monoclonal antibody (mAb) RS7 (antiepithelial glycoprotein-1) was evaluated in a human nonsmall cell lung carcinoma xenograft model. The potential of 177Lu-labeled RS7 was compared with that of RS7 labeled with 90Y and a residualizing form of 131I. Methods: A 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA) conjugate of RS7 was used for radiolabeling with 177Lu-acetate or 88/90Y-acetate. Biodistribution and therapy studies were conducted in nude mice with subcutaneous Calu-3 xenografts. Therapy studies were performed using the maximal tolerated doses (MTDs) of 90Y-DOTA-RS7 (3.9 MBq [105 μCi]) and 177Lu-DOTA-RS7 (10.2 MBq [275 μCi]) and compared with the data obtained using the MTD (13.0 MBq [350 μCi]) of a residualizing form of 131I-RS7. Results: Radiolabeling of RS7-DOTA conjugate with 177Lu-acetate was facile. 177Lu-DOTA-RS7 displayed biodistribution results that were nearly identical to that of the 88Y analog in a paired-label study. The mean percentage injected doses per gram (%ID/g) for 177Lu-RS7 and 88Y-RS7 (in parentheses) in tumor were 38.3 %ID/g (39.1 %ID/g), 63.0 %ID/g (66.0 %ID/g), 63.0 %ID/g (65.8 %ID/g), and 34.0 %ID/g (34.9 %ID/g) on days 1, 3, 7, and 14, respectively. Elimination of established tumors, with an initial mean tumor volume of 0.24 cm3, was shown using doses of 177Lu-DOTA-RS7 ranging from 5.6 to 9.3 MBq (150–250 μCi) per nude mouse, with no significant difference in response rate noted between the doses in this range. Specificity of the therapeutic effect was shown in an isotype-matched control experiment, in which 177Lu-DOTA-RS7 was markedly more effective than the 177Lu-DOTA control antibody. A comparison of the therapeutic efficacies of 177Lu-DOTA-RS7 and 90Y-DOTA-RS7, using mice with established tumors with an initial mean tumor volume of 0.85 cm3, indicated similar tumor growth inhibition and similar tumor regrowth profiles. The therapy data were similar to those obtained with residualizing 131I-RS7 obtained at the same time. Conclusion: 177Lu-RS7 is an effective radioimmunoconjugate for radioimmunotherapy. With its radiophysical properties similar to those of 131I, coupled with its facile and stable attachment to mAb, 177Lu promises to be an alternative to 131I, and a complement to 90Y, in radioimmunotherapy.
Monoclonal antibody (mAb) RS7, an IgG1 murine mAb, reacts with epithelial glycoprotein-1 (EGP-1), an integral membrane glycoprotein (1). A high frequency of EGP-1 expression has been observed in a variety of tumor types (including tumors of the lung, stomach, bladder, breast, ovary, uterus, and prostate), with limited expression on normal human tissue (2). In addition, RS7 is rapidly internalized after binding to target cells (2). Localization and therapy studies using radiolabeled RS7 in animal models have shown tumor targeting and significant antitumor efficacy (3–7).
Initial evaluations of the efficacy of radiolabeled mAbs, including RS7, for cancer therapy were performed with isotopes of iodine as the source of radioactivity. It was realized, however, that the choice of radionuclide is an important issue and that 131I-labeled mAbs had some drawbacks. Specifically, the catabolism of iodinated proteins results in the generation of iodotyrosine within lysosomes and its subsequent release from the cell leads to a shortened residence of the isotope at the target site (8,9). This contrasts with the fate of radiometal chelate-labeled proteins, in which the radiometal remains trapped inside the lysosomes after catabolism (10–12), resulting in higher tumor uptake and longer retention in the tumor. Residualizing forms of radioiodine, which lengthen the residence time of radioiodine at target cells, have been described recently. Although residualizing iodine radiolabels have been shown to accumulate within tumor cells to a greater extent than conventional iodine labels (4,5,12–17), these labels have not yet become widely used because of the complex labeling procedures, low level of radioiodine incorporation, low specific activity, and problems with mAb aggregation. We recently reported that a radioiodinated, diethylenetriaminepentaacetic acid (DTPA)-appended peptide, designated IMP-R1 (maleimidomethylcyclohexlycarbonyl-glycyl-d-tyrosyl-d-lysine [CSNH-benzyl-DTPA]-OH), was a residualizing iodine label that overcame many of the limitations that have impeded the development of residualizing iodine for clinical use (18–20). Using IMP-R1, residualizing 131I-labeled mAbs can be prepared at sufficient yields and specific activities without aggregation or loss of immunoreactivity and that are able to deliver a greatly elevated radiation dose to tumors.
177Lu is a reactor-produced radionuclide that has favorable radiophysical properties, such as moderate 496-keV maximum β-emissions and low-abundance 208-keV γ-emissions and a 6.7-d half-life, resembling 131I. Yet, in its chemistry, it resembles the 90Y nuclide and is a residualizing metallic radionuclide. Two preclinical reports using this nuclide for radioimmunotherapy have appeared (21,22). We have recently extended the short-duration, facile, and near-quantitative 90Y labeling of mAb-(1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid) ([DOTA] Macrocyclics, Inc., Richardson, TX) conjugates (23) to very stable 177Lu labeling of mAbs (24). Because the half-life, β-emission energy, and imagable γ-energy of 177Lu resemble those of 131I, 177Lu represents an attractive alternative to radioiodine. Unlike with radioiodine, no special design of residualizing nuclide would be necessary when using 177Lu with internalizing mAbs because this nuclide residualizes after internalization due to its attachment to the DOTA component of mAb-DOTA conjugates. A comparison of the radiophysical properties of the 3 therapeutic isotopes—177Lu, 90Y, and 131I—is given in Table 1.
Selected Isotopes for Antibody Therapy
In previously reported studies, both 90Y-RS7 and RS7 labeled with residualizing 131I were shown to have a therapeutic advantage compared with the same mAb labeled with 131I by the conventional chloramine-T methodology. Using 90Y-labeled mAb at its maximal tolerated dose (MTD), 50% of the tumor-bearing mice experienced complete remissions, whereas only a 6-wk growth delay occurred after treatment with an equitoxic dose of the conventionally 131I-labeled mAb (6). In another study, using residualizing iodine-labeled RS7, elimination of 6 of 9 established tumors was observed using a single dose of 13.0 MBq 131I-IMP-R1-RS7 per mouse (350 μCi per mouse), with all animals tolerating the dose. At the same dose and specific activity of 131I-RS7, labeled using the chloramine-T method, there were 4 deaths, apparently from a radiation dose that was too high, and 1 complete remission in 9 treated mice. At the MTD of conventionally labeled 131I-RS7, 10.2 MBq (275 μCi), mean stable disease was observed for approximately 5 wk, with no complete responses (20).
The purpose of this investigation was to compare the potential of 177Lu-RS7 with that of RS7 labeled with 90Y and 131I in preclinical targeting and therapy studies. Using a model system comprised of human nonsmall cell lung cancer xenografts in nude mice, the biodistribution of 177Lu-RS7 was compared with that of 90Y-RS7 and comparative toxicities and therapeutic efficacies of 177Lu-RS7, 90Y-RS7, and 131I-IMP-R1-RS7 were determined. We show that in addition to 90Y-RS7 and 131I-IMP-R1-RS7, 177Lu-RS7 is an effective agent for tumor targeting and radioimmunotherapy.
MATERIALS AND METHODS
mAbs and Cell Lines
The production and characterization of mAbs has been described. mAb RS7, an IgG1 murine mAb, reacts with the integral membrane glycoprotein EGP-1 (1). The anticarcinoembryonic antigen (CEA) mAb used in these studies was MN-14, which is directed against the class III, CEA-specific epitope (25). Antibodies were purified from ascites fluid by passage through a protein A-immunoadsorbent column.
Calu-3, a human adenocarcinoma of the lung cell line, was purchased from the American Type Culture Collection (Rockville, MD). The cells were grown in RPMI medium 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mmol/L).
Radiolabeling
The DOTA conjugate preparation was performed as detailed in a published method (23). Briefly, RS7 IgG was coupled to a 90-fold molar excess of in situ–generated monosuccinimidyl DOTA (prepared from DOTA) at pH 8.3–8.5, 4°C, 18 h. The DOTA conjugate of RS7 was determined to contain a DOTA molar substitution ratio of 2.7 by a metal-binding assay (26). This material was used for both small-scale (biodistribution studies) and large-scale (MTD and therapy studies) 177Lu and 90Y labeling using the procedures developed earlier (23,24). The procedure involved aliquoting the required megabecquerels of 177Lu-chloride (Missouri University Research Reactor, Columbia, MO) or 90Y-chloride (NEN Life Science Products, Boston, MA), buffering with 6 times the volume of 0.25 mol/L ammonium acetate, pH 5.4, and adding the requisite megabecquerels of 177Lu-acetate or 90Y-acetate to RS7-DOTA conjugate solution to achieve a specific activity of 74–111 MBq/mg (2–3 mCi/mg). The labeling mixture was heated at 45°C for 5–15 min. The labeling was quenched with 10 mmol/L diethylenetriaminepentaacetic acid (DTPA) and heated for a further 15 min. Radiolabeling efficiency (90% minimum) was analyzed by instant thin-layer chromatography and high-performance liquid chromatography (HPLC). For biodistributions, 88Y was used in place of 90Y, and the labeling was done at a specific activity of approximately 3.7 MBq/mg (0.1 mCi/mg) using buffered 88Y-chloride (Los Alamos National Laboratory, Los Alamos, NM). Aggregation of 177Lu-DOTA-RS7 and 88/90Y-DOTA-RS7 preparations was assessed by HPLC and ranged from 0% to 3%.
Synthesis and radioiodination of IMP-R1, the DTPA-appended peptide used for introduction of residualizing iodine, and conjugation of radioiodine-labeled IMP-R1 to disulfide-reduced mAb have been described (18).
Immunoreactivity
Assessment of immunoreactivity after radiolabeling was performed using a direct cell-binding assay (7). The percentage immunoreactivity, calculated according to Lindmo et al. (27), of the radiolabeled RS7 preparations for each study reported was as follows. In the biodistribution study, immunoreactivities of 177Lu-RS7 and 88Y-RS7 were 77.4% and 100%, respectively. In the MTD, specificity, and comparative therapy studies, immunoreactivities of 177Lu-RS7 were 82.9%, 29.2%, and 76.0%, respectively. Immunoreactivities of 90Y-RS7 and 131I-IMP-R1-RS7 used in the comparative therapy study were 55.8% and 67.6%. Although the reason for the low immunoreactivity determined for the 177Lu-RS7 preparation used in the specificity study is not known, results of this experiment indicate that the preparation was of sufficient quality to effectively cause tumor regression.
In Vivo Studies
Tumors were propagated in female nu/nu mice (Taconic Farms, Germantown, NY) at 6–8 wk of age by subcutaneous injection of 2 × 107 Calu-3 cells, which had been propagated in tissue culture. The mice were used for in vivo biodistribution studies approximately 3 wk after the injection of cells, when tumors reached a weight of approximately 0.2 g (generally in the range of 0.1–0.5 g). Radioiodinated antibodies were injected intravenously, into the lateral tail vein, into the tumor-bearing animals. Details on the quantities of radioisotope injected are indicated in the Results for each study. For biodistribution studies, the animals were killed at the times indicated and the radioactivities in the tumor, liver, spleen, kidneys, lungs, stomach, small and large intestines, muscle, bone (whole femur), and blood were determined after correction for physical decay in a γ-scintillation counter. Results are given as the mean ± SD of 6 animals per time point. For MTD and radioimmunotherapy experiments, tumor size was monitored by weekly measurements of the length, width, and depth of the tumor using a caliper. Weekly follow-up continued for 15–25 wk. Tumor volume was calculated as the product of the 3 measurements. In all studies there was only 1 tumor per animal. The MTD was defined as the highest dose that allows 100% of the animals to survive with no more than 20% loss in body weight. Reversible myelotoxicity was acceptable. Studies were performed using 8–14 animals per group. An untreated group is included in each experiment to assess interexperimental variability of tumor growth. Toxicity was monitored principally by loss of body weight and white blood cell (WBC) counts.
Dosimetry
Radiation dose estimates delivered to the tumor and normal organs were calculated from the biodistribution data, as described (4). Briefly, radiation dose estimates were determined by first integrating the trapezoidal regions defined by the time–activity data (corrected for physical decay). A zero-time value of zero is assumed for the trapezoidal fit. The resulting integral for each organ is converted to centigray per megabecquerel using S values calculated for each isotope by assuming uniformly distributed activity in small unit-density spheres, which do not assume 100% absorption of β-particles (28). For the blood, the absorbed dose was calculated for a sphere the size of the total blood volume of the mouse (assumed to be 1.5 mL). This model of the blood is useful in that the blood dose calculated has been found to correlate well with experimentally determined bone marrow toxicity, which is the dose-limiting toxicity (29). The dose to bone marrow was calculated using a red marrow-to-blood activity concentration ratio of 0.36 (30,31).
Statistical Analyses
Statistical analyses were performed to compare different treatment groups on the basis of 2 variables: area under the growth curve and survival time. The t test was used to analyze the first variable, whereas the log-rank test (32) was used to analyze the second one. The endpoint of the survival time is taken as either the death of the animal or the time at which the tumor reaches a volume of 2.0 cm3. Two-sided tests were used throughout.
RESULTS
Comparative Biodistribution of 177Lu-RS7 and 88Y-RS7 in Calu-3–Bearing Nude Mice
The in vivo targeting of 177Lu-RS7 and 88Y-RS7 was compared in a paired-label biodistribution study in nude mice bearing xenografts of Calu-3 human lung tumors. 88Y was used in the biodistribution experiment as a surrogate for 90Y, the isotope that would be used therapeutically. This substitution was made because the γ-emissions of 88Y enable counting the tissues in the γ-counter. The Calu-3–bearing nude mice were administered intravenously a mixture of 37 Bq (1 μCi) 88Y-DOTA-RS7 and 555 Bq (15 μCi) 177Lu-DOTA-RS7 and killed successively on days 1, 3, 7, and 14. Radioactivities of the tumor, organs, and blood were counted. The percentage injected dose per gram (%ID/g) localizing in the tumor and organs is summarized in Figure 1. The accretion of the 2 isotopes in tumor and normal organs was nearly identical. The mean %ID/g values in tumor for 177Lu were 38.3 ± 5.1 %ID/g, 63.0 ± 15.0 %ID/g, 63.0 ± 6.9 %ID/g, and 34.0 ± 22.7 %ID/g on days 1, 3, 7, and 14, respectively, compared with 39.1 ± 5.4 %ID/g, 66.0 ± 15.6 %ID/g, 65.8 ± 7.2 %ID/g, and 34.9 ± 23.5 %ID/g for 88Y-RS7 at these time points. These levels of 177Lu and 88Y accretion in tumor are markedly higher than published values for the tumor accretion after administration of conventional 131I-RS7 in this tumor model (3,4,19).
Biodistribution of radiolabeled RS7 in nude mice bearing Calu-3 tumors. Mice with tumors were injected intravenously on day 0 with 555 Bq (15 μCi) 177Lu-RS7 (solid line, solid symbols) and 37 Bq (1 μCi) 88Y-RS7 (dashed line, open symbols) and killed successively. Tumor, organ, and blood radioactivities were counted. %ID/g of tissue was calculated from these data. Points represent mean %ID/g of tissue. Error bars represent SDs. Data are shown for tumor (▴, ▵), liver (▪, □), muscle (⧫,◊), and blood (•, ○).
MTD and Dosimetry Analyses
The MTD of 177Lu-DOTA-RS7 was determined experimentally by administering increasing doses of the radiolabeled mAb to nude mice. The study was performed in nontumor-bearing nude mice and in nude mice bearing Calu-3 tumors. The mean tumor volume at the time of radiolabeled antibody treatment in the tumor-bearing mice was 0.24 cm3. Radioantibody doses of 3.7–11.1 MBq (100–300 μCi) were administered to the nontumor-bearing mice in increments of 1.85–2.78 MBq (50–75 μCi), and doses of 5.55–11.1 MBq (150–300 μCi) were administered in 1.85-MBq (50 μCi) increments to groups of 8 or 9 tumor-bearing animals. In each study, 1 animal died 3 wk after treatment in the group that received 11.1 MBq (300 μCi). All lower doses were tolerated with <5% mean loss of body weight. A marked therapeutic effect was observed in the tumor-bearing mice (Fig. 2). Elimination of the Calu-3 tumors was found at all doses of 177Lu-DOTA-RS7, with no significant difference in response rate noted between the doses in this range. At 14 wk after radioimmunotherapy, 11 of 25 animals with established tumors at the time of treatment experienced complete remissions. The remaining 14 animals had partial responses with the remaining tumors averaging 82% reduction in volume. In subsequent studies, 10.2 MBq (275 μCi) 177Lu-DOTA-RS7 was tolerated and this level was used as the MTD.
MTD assessment. Tumor-bearing mice were given single injection of 177Lu-RS7, in increments of 1.85 MBq (50 μCi). Groups consisting of 8 or 9 mice received 5.55 MBq (150 μCi) (•), 7.4 MBq (200 μCi) (▪), 9.3 MBq (250 μCi) (▴), or 11.1 MBq (300 μCi) (×) 177Lu-RS7 per mouse. Mean tumor volume at time of treatment was 0.24 cm3. Inset indicates number of mice obtaining complete response (CR)/total number of mice in each group. Points represent mean tumor volume in treatment groups.
To estimate relative absorbed radiation doses to the tumor and normal organs, dosimetry calculations were performed. Cumulative absorbed radiation doses attributed to 177Lu and 90Y were calculated from the biodistribution data shown in Figure 1. The mean cumulative absorbed doses were computed for 90Y using the 88Y data. Table 2 shows the results of these calculations recorded as centigray per megabecquerel and as the centigray dose to tissues at the experimentally determined MTD values, 10.2 MBq (0.275 mCi) of 177Lu-RS7 per nude mouse and 3.9 MBq (0.105 mCi) of 90Y-RS7 per nude mouse (20). At the MTD levels, cumulative absorbed doses of 7,691 cGy to tumor and 1,860 cGy to blood are calculated for 177Lu-RS7 compared with 4,866 cGy to tumor and 2,460 cGy to blood for 90Y-RS7. Absorbed doses to the normal organs are well below the toxic levels in all cases.
Dosimetry Comparison of 177Lu-RS7 and 90Y-RS7 in Calu-3–Bearing Nude Mice
Specificity of 177Lu-RS7 Radioimmunotherapy
To study the specificity of the therapeutic effect of 177Lu-RS7, tumor growth was followed in Calu-3–bearing nude mice after treatment with 10.2 MBq (275 μCi) of either 177Lu-RS7 or a 177Lu-labeled negative control antibody. Anti-CEA mAb MN-14, a murine IgG1, was used as a negative control because it is the same isotype as RS7 and the Calu-3 cell line does not express CEA (7). The mean tumor volume at the time of radiolabeled antibody treatment in this study was 0.43 cm3. As shown in Figure 3, 177Lu-RS7 was significantly more effective than the 177Lu-labeled control antibody, indicating specificity of the therapeutic response. A t test comparison of the area under the curves of mean tumor volume (Fig. 3A) yielded P < 0.05. The survival curves (Fig. 3B) were also significantly different (P < 0.02). Complete remissions were seen in 5 of 8 mice treated with 177Lu-RS7; the remaining animals had partial remissions. Reductions of 93% and 95% in the mean tumor volume were observed at 10 and 20 wk after treatment, respectively. Of the mice treated with the control mAb, 177Lu-MN-14, 2 of 7 had complete remissions. However, by 10 wk after treatment, tumors in 4 of the 7 mice had more than doubled in size, with a mean tumor volume of 6.8-fold greater than the starting volume. At the termination of the study, 20 wk after treatment, the mean tumor volume was >9-fold greater than the starting volume in the 177Lu-MN-14 treatment group.
Specificity of radioimmunotherapy with 177Lu-labeled mAbs in Calu-3–bearing nude mice. Tumor-bearing mice were given single injection of 10.2 MBq (275 μCi) 177Lu-RS7 (•, n = 8) or 177Lu-MN-14 (○, n = 7). Mean tumor volume at time of treatment was 0.43 cm3. (A) Points represent mean tumor size of animals in treatment groups. Error bars represent SDs and in upper curve are shown only above symbol for clarity. (B) Points represent percentage of animals surviving, defined as either time of death of animal or time at which tumor reaches volume of 2.0 cm3.
Comparison of Therapeutic Efficacy of Isotopes in Calu-3–Bearing Nude Mice
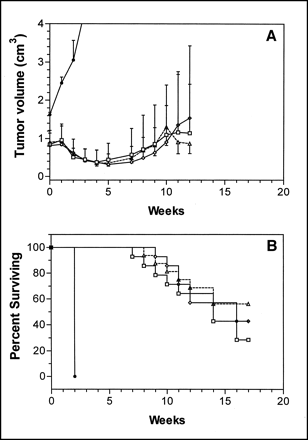
A direct comparison of the therapeutic efficacy and toxicity of the MTDs of 131I-IMP-R1-RS7 (13.0 MBq [350 μCi]), 177Lu-RS7 (10.2 MBq [275 μCi]), and 90Y-RS7 (3.9 MBq [105 μCi]) was performed in this tumor model using mice with large established tumors (mean tumor volume, 0.85 cm3) at the time of treatment. Mean tumor growth and survival curves are shown in Figure 4. Antitumor efficacy of the 3 treatments was comparable. In the 3 treatment groups, mean tumor volume regression was observed for 5–6 wk after treatment, and the mean tumor volume before treatment was reached at 9–10 wk after radioimmunotherapy. Objective response rates were also similar between the groups. Complete responses, partial responses, and stable disease in various groups were 2, 10, and 2, respectively, with 90Y; 1, 10, and 3, respectively, with 177Lu; and 2, 9, and 3, respectively, with 131I. No statistical differences were observed in either the area under the growth curve or the survival analyses. The treatment groups also experienced equivalent toxicity as measured by changes in body weight and WBC counts. As shown in Figure 5, nadirs of 47%–50% and 22%–30% of the control levels for total WBC and lymphocyte counts, respectively, were observed 1–4 wk after radioimmunotherapy. As observed previously after radioimmunotherapy (33), the effect on lymphocytes is more severe in terms of both the level and the duration of the effect in comparison with the effect on neutrophils.
Comparative radioimmunotherapy in Calu-3–bearing nude mice using 177Lu-RS7, 90Y-RS7, and 131I-IMP-R1-RS7 at respective MTD levels. Tumor-bearing animals were given single injection of 13.0 MBq (350 μCi) 131I-IMP-R1-RS7 (□), 3.9 MBq (105 μCi) 90Y-RS7 (◊), or 10.2 MBq (275 μCi) 177Lu-RS7 (▵) or were left untreated (•). (A) Points represent mean tumor size of living animals in treatment groups. Error bars represent SDs and are shown only above symbol for clarity. (B) Points represent percentage of animals surviving, defined as either time of death of animal or time at which tumor reaches volume of 2.0 cm3. 131I-IMP-R1-RS7 and 90Y-RS7 data are adapted from (20).
Toxicity evaluations after radioimmunotherapy. Total WBCs (A), lymphocytes (B), and neutrophils (C) were evaluated weekly in mice shown in Figure 4. Blood cell counts are presented as percentage of untreated group. Tumor-bearing animals were treated with 13.0 MBq (350 μCi) 131I-IMP-R1-RS7 (□), 3.9 MBq (105 μCi) 90Y-RS7 (◊), or 10.2 MBq (275 μCi) 177Lu-RS7 (▵). Data points represent means of animals shown in Figure 4. Error bars represent SDs and are shown only in 1 direction for clarity.
DISCUSSION
These studies show that in addition to 90Y-RS7 and RS7 labeled with residualizing 131I, 177Lu-RS7 is an effective agent for tumor targeting and radioimmunotherapy. The biodistribution of 177Lu-RS7 was equivalent to that of 88Y-RS7, with both radiometals yielding markedly higher accretion in tumor than published values for conventional 131I-RS7 in this tumor model (3,4,19). The mean %ID/g in tumor 7 d after administration of 131I-RS7 ranged from 5.5 %ID/g to 8.6 %ID/g compared with >60 %ID/g in tumor 7 d after administration of 177Lu- and 88Y-RS7 in this study. The therapeutic efficacy and toxicity of 177Lu-RS7 in large established human tumor xenografts were equivalent to those of residualizing 131I-RS7 and 90Y-RS7. Objective response rates achieved using RS7 labeled with all 3 isotopes were similar, with 1 or 2 complete responses, 9 or 10 partial responses, and 2 or 3 animals with stable disease per group in the animals with large established tumors. An increased effectiveness of 177Lu-RS7 was observed with the somewhat smaller established tumors used in the MTD and specificity studies (0.24 and 0.43 cm3, respectively). In these experiments, radioimmunotherapy with 177Lu-RS7 yielded a 50% complete response rate. Specificity of the therapeutic effect was shown in an isotype-matched control experiment, in which 177Lu-DOTA-RS7 was markedly more effective than the 177Lu-DOTA control antibody MN-14.
Improvements in the tumor accretion of radiolabel have been seen previously with residualizing isotopes, including residualizing iodine using 125I-dilactitoltyramine (DLT)-RS7 and 125I-IMP-R1-RS7, in comparison with the conventional 131I-labeled antibody (4,19). The %ID/g in tumor at day 7 for these residualizing forms of radioiodine in this model were 18.1 %ID/g and 38.1 %ID/g for 125I-IMP-R1-RS7 and 125I-DLT-RS7, respectively. Because 125I-IMP-R1-RS7 cleared more rapidly from the blood than conventionally iodinated RS7, the 131I-IMP-R1-RS7, as well as the 131I-DLT-RS7, yielded a 4- to 5-fold increased cumulative absorbed dose to tumor, when normalized to the blood dose.
Dosimetry calculations based on the radiophysical properties of the isotopes, including the longer half-life of 177Lu relative to 90Y, and the nearly identical biodistribution of mAb labeled with the 2 isotopes predicted that 177Lu-labeled mAbs should be able to deliver higher doses to tumor at the MTD. In addition, we previously predicted that residualizing 131I would deliver a 40% higher dose to tumor at the calculated MTD than would 90Y (19). However, our therapy study shows an equivalent efficacy of equitoxic doses of the 3 isotopically labeled forms of RS7 in large established tumors. This apparent discrepancy could be attributed to inaccuracies of the dosimetric model used as well as an inability of the murine model to expose therapeutic differences that could become apparent in the clinical setting. The higher calculated blood dose at the experimental MTD for 90Y-RS7 most likely reflects an overestimation of the dose to blood because the dosimetry model we used for calculating blood dose assumes all organs (including blood) to be uniform-density spheres. This assumption artificially elevates the calculated dose to blood for longer path-length isotopes, such as 90Y, where a large amount of the dose attributed to blood is actually deposited outside the blood vessels. For the tumor and normal organs, the spheric model is closer to the true geometry of the tissue; thus, radiation dose estimates are more accurate.
177Lu has been used in preclinical (21) and clinical (34,35) radioimmunotherapy with the anti–tumor-associated glycoprotein-72 mAb, CC49. In these studies, the radiolabeling was done as a multistep process involving 177Lu labeling of a bifunctional chelating agent, 1,4,7,10-tetraaza-1-(1-carboxy-3-(4-aminophenyl)propyl)-tris-4,7,10-car-boxymethyl)cyclododecane, followed by activation with thiophosgene, purification, conjugation to mAb CC-49, and final purification of the radiolabeled antibody. We have developed a simpler method for radiolabeling DOTA conjugates of antibodies with radioyttrium (23,36) and radiolutetium (24). This involves simply mixing a preformed mAb-DOTA conjugate with 88Y-acetate, 90Y-acetate, or 177Lu-acetate to the desired specific activity and heating for 5–30 min at 45°C to obtain >90% radiolabel incorporation with high chelate stability. The simplified radiolabeling procedure gives an added impetus to the development of 177Lu-labeled mAbs for eventual clinical radioimmunotherapy applications. More recently, 177Lu has been found to be a promising radionuclide in the preclinical radionuclide therapy of somatostatin receptor–positive tumors (37,38).
The radiophysical properties of 177Lu yield several advantages compared with 131I and 90Y for use as a radioimmunoconjugate for the treatment of tumors. The isotopes with longer half-lives may be advantageous in terms of marrow toxicity because less of the decay occurs during the early time period when the blood contains a higher percentage of the injected dose of the isotope and less of the dose has accumulated at the tumor target. An equally important consideration is that a greater fraction of the lifetime of the isotope remains during the later time period when the radioimmunoconjugate has accumulated at the tumor. Another advantage of 177Lu in comparison with 131I and 90Y is the emission of moderate-energy γ-rays. The higher energy, more abundant γ-rays emitted by 131I may contribute to patient toxicity, pose a greater radiation hazard to health care workers, and necessitate a longer hospital stay. In contrast, 90Y’s lack of γ-rays is a disadvantage because as a pure β-emitter it cannot be used for imaging.
Because of the shorter path lengths of 177Lu and 131I relative to 90Y, it is possible that these isotopes might be more effective than 90Y for smaller tumors, where more of the dose would be absorbed by the cancer cells, rather than surrounding normal tissue. It is likely that 177Lu-RS7 and 131I-RS7 might be more effective than 90Y-RS7 for micrometastatic disease or in an adjuvant setting, whereas 90Y would be more useful for bulky disease where its longer path length would be an advantage. Clinical trials will be necessary to determine which of these 3 radioisotopes will yield the most effective agent for radioimmunotherapy in patients in various disease settings.
CONCLUSION
177Lu-RS7 is an effective radioimmunoconjugate for radioimmunotherapy of even relatively large tumor xenografts, as seen in this study. With its radiophysical properties similar to those of 131I, coupled with its facile and stable attachment to mAb, 177Lu promises to be an alternative to 131I, and a complement to 90Y, in radioimmunotherapy.
Acknowledgments
The authors thank Philip Andrews and Thomas Jackson for radiolabeling and quality control. This project was supported in part by U.S. Public Health Service grant CA60039 and Small Business Innovation Research Program grant CA72324. This research was presented in part at the 47th Annual Meeting of the Society of Nuclear Medicine, St. Louis, MO, June 2000.
Footnotes
Received Oct. 20, 2000; revision accepted Feb. 15, 2001.
For correspondence or reprints contact: Rhona Stein, PhD, Garden State Cancer Center, 520 Belleville Ave., Belleville, NJ 07109.