Abstract
111In-Labeled antibodies and peptides have been routinely used as chemical and biologic surrogates for 90Y-labeled therapeutic agents. However, recent studies have shown that there are significant differences in biodistribution between 111In- and 90Y-labeled agents. Yttrium and lutetium metals favor the +3 oxidation state, similar to indium, but there are minor differences in the solution and coordination chemistries among these metals. These 3 metals, however, form strong complexes with the macrocyclic chelator, 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (DOTA). We, therefore, compared the pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody. The radiation dosimetry of 90Y-J591 was estimated based on both 111In and 177Lu data to validate the usage of 111In as a chemical and biologic surrogate for 90Y. Methods: J591 is a deimmunized monoclonal antibody with specificity for the extracellular domain of prostate-specific membrane antigen. In patients with prostate cancer, phase I dose-escalation studies were conducted with 90Y-J591 (n = 29) and 177Lu-J591 (n = 25). Each patient had pharmacokinetics and imaging studies with 111In-J591 (185 MBq/20 mg) over a period of 1 wk and before treatment with 90Y-J591 antibody. In the 177Lu trial, the pharmacokinetics and imaging studies were performed after treatment with the 177Lu-J591 dose (370–2,590 MBq/m2/10 mg/m2) over a 2-wk period after treatment. Results: Blood and urinary pharmacokinetics were similar for both tracers. Based on biexponential decay, the terminal half-life was 44 ± 15 h for both tracers. In addition, the total-body retention of radioactivity over a 7-d period was also similar between the 2 isotopes. The percentage uptake in liver was about 20% greater with 111In than with 177Lu. Radiation dosimetry estimates for 90Y-J591 calculated on the basis of 111In or 177Lu data were mostly similar and showed that liver is the critical organ, followed by spleen and kidney. Based on blood radioactivity, the radiation dose (mGy/MBq) to the bone marrow was 3 times higher with 90Y (0.91 ± 0.43) compared with that with 177Lu (0.32 ± 0.10). Conclusion: 111In- and 177Lu-labeled J591 antibodies have similar plasma and whole-body clearance kinetics. The net retention of 111In activity by lung, liver, and spleen is slightly higher compared with that with 177Lu. These results justify using 111In as a chemical and biologic surrogate for 90Y. However, the radiation dose to the liver may be overestimated by about 25% based on 111In data. In addition, the data also suggest that 177Lu may be a potential alternative for estimating the pharmacokinetics and biodistribution of 90Y-labeled radiopharmaceuticals.
In recent years targeted radioimmunotherapy (RIT) using monoclonal antibodies (mAbs) directed to cancer-related, cell-surface antigens has been clinically validated. 90Y- and 131I-labeled anti-B1 mAbs have shown 40%–70% antitumor response in patients with lymphoma and are approved by the Food and Drug Administration (1,2) for the treatment of patients with low-grade, non-Hodgkin’s follicular lymphoma. 131I and 90Y have emerged as the primary choices for RIT; however, these 2 nuclides have potential advantages and disadvantages. 131I has lower energy β−-particles and a longer physical half-life (maximum β−, 0.61 MeV; t1/2 = 8.04 d) compared with that with 90Y (maximum β−, 2.28 MeV; t1/2 = 2.67 d). The radioiodinated mAb is dehalogenated in vivo, and the free radioiodide and the iodinated peptide fragments are washed out of tissues and excreted in the urine. In contrast, when 90Y is bound to mAbs via a bifunctional chelate, the radiolabeled antibody complex is stable in vivo and 90Y is trapped within the cell, leading to higher accretion by the tumor. 131I has γ-photons (0.364 MeV) useful for biodistribution and dosimetry studies. Since 90Y does not emit γ-photons, 111In-labeled antibodies are generally used as chemical and biologic surrogates (3,4) to study biodistribution and estimate radiation dosimetry of 90Y-labeled antibodies. Some recent studies, however, have shown that there are significant differences in biodistribution between 111In- and 90Y-labeled agents (5).
In addition to 90Y, other β−-emitters—such as the lanthanide radiometals 177Lu (maximum β−, 0.497 MeV; t1/2 = 6.74 d) and 166Ho (maximum β−, 1.84 MeV; t1/2 = 1.12 d)—are also potentially useful for RIT. Unlike 90Y, both 177Lu (γ = 0.21 MeV) and 166H (γ = 0.21 MeV) have useful γ-photons for biodistribution and dosimetry studies. Chemically, yttrium and lutetium metals favor the +3 oxidation state, similar to indium, but there are minor differences in the solution and coordination chemistries among these metals (6–8). Preclinical studies have shown that the in vivo behavior of 90Y-labeled mAb is much more similar to that of 177Lu-labeled antibody compared with that with 111In-labeled antibody (9). Therefore, comparison of the pharmacokinetics and biodistribution of 111In- and 177Lu-labeled antibodies in human subjects would provide a better understanding of the in vivo behavior of mAbs labeled with these radiometals.
The process of developing successful radiolabeled antibodies for cancer RIT requires identification of a cancer-restricted, cell-surface antigen and development of an antibody specific for that target molecule. The most well-established, prostate cancer–restricted cell-surface antigen identified to date is prostate-specific membrane antigen (PSMA) (10). It is an ideal target for RIT, since it is a highly prostate-restricted, type II integral membrane cell-surface glycoprotein expressed by all prostate cancers (11,12), and expression levels progressively increase in more poorly differentiated, metastatic, and hormone-refractory cancers (13,14). J591 is an anti-PSMA mAb that binds with high affinity to the extracellular domain of PSMA and is rapidly internalized (15–17). We have recently reported excellent tumor targeting of radiolabeled J591 mAb in patients with prostate cancer (18).
We report here the pharmacokinetics and biodistribution data of 2 independent phase I dose-escalation clinical studies with J591 mAb labeled with 90Y or 177Lu. We compared the pharmacokinetics and biodistribution of 111In-DOTA-J591 (DOTA = 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid) (111In-J591) mAb with that of 177Lu-DOTA-J591 (177Lu-J591) mAb (19). In addition, we have also compared the radiation dosimetry estimates of 90Y-DOTA-J591 (90Y-J591) based on either 111In or 177Lu studies.
MATERIALS AND METHODS
Patient Population
Eligible patients had a prior histologic diagnosis of prostate cancer with evidence of recurrent or metastatic disease as defined by an increasing PSA level or abnormal radiologic studies, including bone scan, axial CT, or MRI. Patients were required to have a PSA level of ≥1.0 at the time of entry with 3 consecutive increasing PSA values over a period of ≥2 wk. Additional requirements included a platelet count of ≥150,000/mm3 and a neutrophil count of ≥2,000/mm3 and a bone marrow biopsy demonstrating ≤10% replacement by tumor on a unilateral sample or a mean of ≤25% replacement by tumor on bilateral samples.
Antibody
Murine J591 mAb was deimmunized by Biovation, Ltd. The deimmunization involves removal of mouse amino acid sequences and replacement with homologous human, nonimmunogenic sequences (20). Clinical grade deimmunized J591 mAb was produced under Good Manufacturing Practice (GMP) conditions at Lonza Biologics, Plc., and supplied in 5 mL of phosphate buffer (pH 7.0) containing 5 mg/mL of antibody. Subsequently, J591 antibody was covalently linked with the chelating agent, DOTA (Goodwin Biotech), as previously reported (17). The sterile pyrogen-free clinical material, DOTA-J591 mAb in 0.3 mol/L ammonium acetate buffer (pH 7.0) (8 mg/mL), was provided by BZL Biologics, Inc.
Radiolabeled Antibodies
111In chloride and 90Y chloride were purchased from Nordion. 177Lu chloride was purchased from the University of Missouri-Columbia Research Reactor Center. The DOTA-J591 mAb was labeled by incubating radiometals in an ammonium acetate buffer with antibody as previously described (17). Radiolabeled J591 mAb was purified by gel filtration and sterilized by membrane (0.2 μm) filtration before administration to patients. The labeling efficiency and radiochemical purity of radiolabeled antibody were determined using Gelman ITLC-SG and 5 mmol/L diethylenetriaminepentaacetic acid (DTPA) solution as the solvent. The immunoreactivity of radiolabeled J591 mAb preparations was determined based on the method of Lindmo et al. (21) using PSMA-positive LNCaP tumor cells.
Dose Escalation and Administration
In a dose-escalation trial with 90Y-J591 (Table 1), patients received 185 MBq of 111In-J591 for pharmacokinetics and biodistribution studies 1 wk before 90Y-J591 administration. After completion of the 111In studies, each patient received the 90Y dose, which was escalated in cohorts of 3–6 patients at the following planned dose levels: 185, 370, 555, 647.5, and 740 MBq/m2 (5–20 mCi/m2). In the dose-escalation trial with 177Lu-J591 (Table 2), patients received 177Lu activity ranging from 370 to 2,775 MBq/m2 (10–75 mCi/m2). Additional unconjugated (unlabeled) J591 antibody was added to give a constant protein dose of 20 mg with the 111In- or 90Y-J591 dose or 10 mg/m2 with the 177Lu dose. The final radiolabeled J591 mAb was diluted to 20 mL with physiologic saline solution and was infused intravenously over a period of 5 min.
Phase I Dose-Escalation Trial with 90Y-J591
Phase I Dose-Escalation Trial with 177Lu-DOTA-J591
Pharmacokinetics
After infusion of the diagnostic dose of 111In-J591 or the treatment dose of 177Lu-J591, venous blood samples were obtained at 10 min, 1, 2, 4, and 24 h, and 2, 3, 4, and 7 d. In the 177Lu protocol, 2 additional samples were obtained during 10–14 d. The radioactivity in 1-mL plasma samples was measured in an automatic γ-counter (MINAXI γ-5550; Packard Instrument Co.) along with a known 111In or 177Lu standard, and the activity in plasma was expressed as the percentage injected dose per milliliter (%ID/mL) The time–activity data were plotted using GraphPad Prism software, and the curves were fitted to mono- and biexponential functions to generate plasma clearance rate constants. Based on the initial (α) and terminal (β) t1/2 and the y-intercepts, several parameters—including the area under the curve (AUC), maximum concentration in plasma (Cmax), volume of distribution (Vd), and clearance rate—were calculated.
Imaging Studies
To assess the biodistribution of J591 mAb, total-body images were obtained within 1 h after infusion (day 0) and again at 4 additional time points in the subsequent week (e.g., 1, 2, 3, and 6–7 d) for 111In and over the next 2 wk (e.g., 1, 3, 6–9, and 13–14 d) for 177Lu. The γ-camera images were obtained using a dual-head ADAC Laboratories or General Electric γ-camera fitted with an appropriate collimator. All 5 scans for each patient were obtained with the same γ-camera. Scan consistency and camera reliability were verified with an 111In (1.85 MBq/20 mL) or a 177Lu (11.1 MBq/20 mL) standard placed between the patient’s legs. The imaging dataset at each time point was corrected for the presence of the standard, background counts, scan speed, and physical decay and then the data were normalized to the day 0 counts. SPECT studies of the abdomen, pelvis, or areas of suspected metastatic lesions were performed on day 2–3 or day 6–7 in selected patients.
Radiation Dosimetry
To determine the biodistribution of radiolabeled antibody, regions of interest (ROIs) were drawn around the major organs (heart, liver, spleen, kidneys, bone marrow, gastrointestinal tract, and bladder) and the whole body. The remainder was defined as whole-body counts minus the sum of counts in the specific ROIs. The data points representing the percentage injected dose (%ID/organ) were created and fitted to a monoexponential, a biexponential, or an uptake-and-clearance curve. After curve fitting and integration, the cumulative activity in each organ and the residence time (τ) for each organ were calculated. The percentage injected dose in blood (plasma) was used to estimate the cumulative activity in bone marrow assuming a ratio of 0.36 for bone marrow to blood (22). The 111In data were used to estimate the residence times for 90Y. The radiation-absorbed doses of 111In-J591 and 90Y-J591 were calculated by entering the corresponding residence times into the MIRDOSE software program (23), which computes the radiation-absorbed dose values as mGy/MBq (or rad/mCi) for each of the target organs. Since the MIRDOSE software did not provide S factors for 177Lu, S factors were initially calculated based on the emission spectrum of 177Lu. Subsequently, the radiation dosimetries of 111In-, 90Y-, and 177Lu-labeled J591 mAb were all calculated based on a new code called OLINDA (Organ Level INternal Dose Assessment), developed at Vanderbilt University. This program performs internal dose calculations, principally for radiopharmaceuticals, using the RADAR (Radiation Dose Assessment Resource) method of dose calculations and RADAR dose factors (24). RADAR is a working group that maintains resources for internal and external dose calculations.
RESULTS
Patients
Sixty-four patients were enrolled in 2 independent phase I dose-escalation trials with 111In/90Y-J591 (n = 29) or 177Lu-J591 (n = 35) between October 2000 and August 2003. We report here the dosimetry data in 27 patients from the 90Y trial and 28 patients from the 177Lu trial. Patients in both groups were 47–85 y old and had prior hormonal therapy, except for 1 patient. In the 90Y study, some patients had prior radiotherapy (62%) or chemotherapy (41%). Similarly, in the 177Lu study, some patients had radiotherapy (46%) or chemotherapy (29%). Most patients (60%) in both groups had bone metastasis based on bone scans, whereas some of them had soft-tissue metastasis identified by CT or MRI.
Radiolabeled Antibodies
With DOTA-J591 mAb, the radiolabeling efficiency of 111In is higher (91% ± 8%) than that with either 177Lu (78% ± 8%) or 90Y (71% ± 8%). However, the radiochemical purity of radiolabeled J591 mAb is similar with 111In (98% ± 2.1%), 177Lu (99% ± 1%), or 90Y (98% ± 1.5%). The specific activity with both 111In-J591 and 90Y-J591 is 111–222 MBq/mg compared with 185–481 MBq/mg with 177Lu-J591. The immunoreactivity with all the 3 preparations is >80% (90 ± 8).
Pharmacokinetics
Pharmacokinetic analysis of plasma samples obtained after the administration of 111In-J591 or 177Lu-J591 mAbs is summarized in Table 3. After intravenous administration, the maximum concentration (Cmax) in plasma (%ID/L) was about 25% for both agents. There is biexponential plasma clearance; <20% of the activity had a fast component with a t1/2 of <3 h. The remaining 80% of both 111In-J591 and 177Lu-J591 activity cleared from plasma slowly, with an average t1/2 of 44 ± 15 h. Based on monoexponential clearance, the t1/2 was a little longer with 177Lu-J591 (39 ± 13) than that with 111In-J591 (32 ± 8), but the difference was not significant (P > 0.05). The other pharmacokinetic parameters, such as AUC, Vd, and clearance, were also similar between these 2 agents.
Plasma Clearance Kinetics: 111In-J591 vs. 177Lu-J591
The kinetics of urinary excretion of 111In and 177Lu activity until 72 h after injection is shown in Figure 1. After administration of radiolabeled J591, the percentage of injected radioactivity in the total urine collected over a period of 3 d is similar for both 111In (6.2% ± 2.4%) and 177Lu (7.3% ± 2.8%).
Urinary excretion of 111In- and 177Lu-labeled J591 mAb over 72-h period from time of administration to patients.
Imaging Studies and Biodistribution
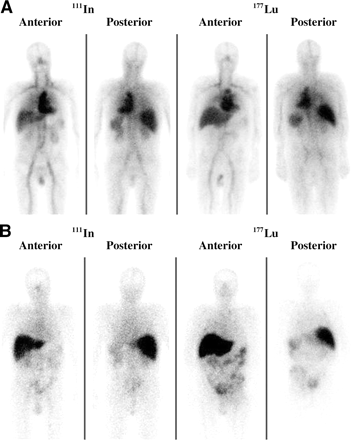
Whole-body γ-camera images comparing the biodistribution of 111In-J591 and 177Lu-J591 are shown in Figures 2A and 2B. With both radionuclides, at 1 and 24 h after injection, radioactivity was predominantly in the blood pool, as seen by the increased activity in the heart and major blood vessels compared with uptake of the radioactivity by the organs. Subsequently, there was a decrease in blood-pool activity with a gradual accumulation of activity in liver, spleen, kidneys, and bone or bone marrow. Starting from day 2, both tracers showed some gastrointestinal activity. Images on day 6–7 clearly showed that 111In and 177Lu were equally effective in identifying the metastatic lesions with a very high target-to-background contrast. For 111In-J591 and 177Lu-J591, the percentage injected dose in several organs at different times after injection are compared in Table 4. For both tracers, the liver accumulated the highest amount of radioactivity and there were minor differences between the 2 radiotracers. Time–activity data in Figure 3 demonstrate that, although the initial liver uptake kinetics were similar for both tracers, the mean liver uptake with 177Lu was about 20% less than that with 111In on day 6 and shows washout of activity on day 13. The whole-body retention of activity, however, was similar for both tracers.
Whole-body images of 111In-J591 and 177Lu-J591 mAb on day 0 (A) and day 6 (B) after administration to 2 different patients.
Whole-body (WB) clearance and liver uptake and washout kinetics of 111In-J591 and 177Lu-J591 mAb from time of administration to patients.
Biodistribution of Radiolabeled J591 mAb in Patients with Prostate Cancer: 111In vs. 177Lu
Radiation Dosimetry
Radiation-absorbed dose estimates (mGy/MBq) for several target organs from 111In-J591 and 177Lu-J591 are summarized in Table 5. For both tracers, the liver received the highest dose, followed by spleen and kidney. Hence, liver is the critical organ, with a radiation-absorbed dose of 1.14 ± 0.89 mGy with 111In and 2.10 ± 0.60 mGy with 177Lu for each megabecquerel of administered dose. For most organs, the dose from 177Lu is about 2–3 times that with 111In.
Radiation Absorbed-Dose Estimates: 111In-J591 vs. 177Lu-J591 mAb
The radiation dosimetry of 90Y-J591 was estimated based on the pharmacokinetics and biodistribution of 111In-J591 and 177Lu-J591 studies and the results are compared in Table 6. The radiation dose (mGy/MBq) to liver was about 26% higher based on the 111In data (6.57 ± 2.27) compared with the value based on the 177Lu data (4.9 ± 1.45). For other major organs, such as spleen, kidneys, and bone marrow, the radiation dosimetry estimates were very close with minimal difference.
Radiation Dosimetry of 90Y-J591
DISCUSSION
PSMA is the most well-established, prostate-restricted, cell-surface antigen identified to date. Hence, it is an excellent target to develop radiolabeled mAbs for the treatment of prostate cancer. To our knowledge, deimmunized J591 mAb is the first radiolabeled antibody specific for the extracellular domain of PSMA to be tested as a radiotherapeutic in patients with prostate cancer. We have previously documented that radiolabeled J591 binds with high affinity (1 nmol/L) to PSMA and that the PSMA-antibody complex is internalized, thereby delivering the radionuclide only to the interior of the targeted cancer cells (16,17). We have also reported previously that radiolabeled J591 specifically and sensitively targets bone and soft-tissue metastatic sites (Fig. 4) in patients with prostate cancer (18). This article reports the biodistribution, pharmacokinetics, and radiation dosimetry of radiolabeled J591 mAb from data obtained in phase I dose-escalation trials.
Whole-body images of 99mTc-methylene diphosphonate (A) and 177Lu-J591 mAb (B) in patient with metastatic prostate cancer. 177Lu images obtained on day 7 show significant localization of radiolabeled J591 mAb in most lesions identified on bone scan (A).
The development of radiolabeled mAbs as therapeutic agents involves the estimation of radiation-absorbed dose as part of the safety assessment in phase I dose-escalation clinical trials. Typically, radiation dosimetry is not part of treatment planning. However, knowledge of the radiation-absorbed doses to various critical organs—especially bone marrow, liver, kidney, and spleen—is crucial for understanding the dose–response relationships of myelotoxicity and second organ toxicities. Quantitative dosimetric imaging and pharmacokinetic studies of the radiolabeled therapeutic agent are essential to accurately measure the time course of radioactivity in organs to calculate residence times, which are used in MIRD schema for estimating the radiation dosimetry. Among the most popular β−-emitters used for radiotherapy, 90Y is the only radionuclide that has no γ-photons for external scintigraphy. The positron emitter 86Y (t1/2 = 14.74 h) may be an appropriate isotope to study the in vivo distribution of 90Y-labeled peptide (4) but is not suitable for dosimetric studies of radiolabeled mAbs because of the relatively shorter physical t1/2 compared with the biologic t1/2 of antibody clearance from circulation. In a small group of patients, the plasma clearance kinetics of 90Y-J591 were similar to those of 111In- and 177Lu-labeled J591 (data not reported). 111In-Labeled agent is generally used as a chemical and biologic surrogate to trace the biodistribution of 90Y-labeled therapeutic agent. However, preclinical and clinical studies have reported the similarities and differences in the biodistribution of 111In- and 90Y-labeled antibodies and challenged the assumption that 111In is a biologic surrogate for 90Y (4,5). Since it has been well documented that the chemistry of yttrium is more similar to that of lutetium (6,7), we hypothesized that 177Lu may be a more appropriate radionuclide to trace the biodistribution of 90Y. This article reports a direct comparison of 90Y dosimetry based on 111In and 177Lu studies in the same patient population.
Pharmacokinetics and Biodistribution: 111In-J591 Versus 177Lu-J591
After intravenous administration, the plasma clearance kinetics of 111In-J591 and 177Lu-J591 from circulation are similar. Between these 2 tracers, no statistically significant differences were observed in the biologic t1/2, AUC, Cmax, Vd, and clearance (Table 3). More than 80% of radiolabeled J591 cleared from blood with a terminal t1/2 (β) of 44 ± 15 h. In the same patient population within a small group (n = 6), 90Y-J591 also had a similar t1/2 of 41 ± 11 h. In the published literature, there is only one other mAb that was labeled with these 3 nuclides. The murine mAb CC49 binds to an epitope of mucin antigen, TAG-72 (25). In patients with adenocarcinomas, the terminal t1/2 for 111In-CC49 was 59.8 h (range, 33–90 h) and for 177Lu-CC49 was 67 ± 15 h (26,27). The 90Y-CC49 had a lower t1/2 of 47.4 h (range, 28–66 h), but the difference was not significant. Both J591 and CC49 antibodies were first conjugated with the chelating agent, DOTA, before labeling with radiometals. Therefore, these 2 studies clearly document that plasma clearance of mAbs is similar when labeled with 111In, 177Lu, or 90Y.
Similarly, the whole-body retention of radiolabeled J591 at 6 d after injection determined on the basis of imaging studies was also similar between 111In (66.1% ± 18%) and 177Lu (69.8% ± 9.5%). In addition, no significant difference was observed between these 2 tracers in the amount of activity excreted in the urine during this time.
The imaging studies, however, showed that there were minor differences (Table 4) in the biodistribution of 111In- and 177Lu-labeled J591. During the first week, both tracers showed a gradual accumulation in the liver and, by day 6, the amount of 111In activity was 25% higher compared with that with 177Lu (P < 0.05). Since 177Lu imaging studies were continued for an additional week, we were able to document that the liver time–activity curve is biphasic. There was significant washout of 177Lu activity from the liver: 24% ± 7% on day 7 compared with 16% ± 5% on day 13 (P < 0.03). Similarly, there were minor, but significant, differences in the spleen, lung, and remainder activities between these 2 nuclides. The biodistribution data with 111In- and 177Lu-labeled CC49 mAb were not available for direct comparison (26,27).
Radiation Dosimetry
For both 111In- and 177Lu-labeled J591 mAb, liver is the critical organ, followed by spleen and kidney (Table 5). The dose (mGy/MBq) to liver with 177Lu (2.10 ± 0.60) was about 80% higher compared with that with 111In (1.14 ± 0.89). Similarly, for all other source organs (spleen, kidneys, lung, heart contents), the dose with 177Lu was 60%–80% higher compared with that with 111In. But for all target organs, the dose with 177Lu was either similar or less than that with 111In. The higher radiation dose to source organs with 177Lu is understandable since the equilibrium dose constant (rad·g/h) for β−-particles is 0.284 with 177Lu compared with 0.117 with 111In. In contrast, the equilibrium dose constant for γ-photons with 111In (0.822) is about 11 times greater compared with that with 177Lu (0.075).
A comparison of radiation dosimetry estimates for Y-J591 based on 111In and 177Lu studies is summarized in Table 6. For most of the source organs, the difference between these 2 estimates was <25%. The dose estimates for liver and lung were significantly higher (20%–25%) with 111In compared with those with 177Lu. This difference can be explained based on the observation that the net retention of 111In activity in the liver and lungs was significantly higher compared with that with 177Lu (Table 4). With 90Y-J591, the estimates for radiation dose to bone marrow were similar based on 111In or 177Lu blood activity. This is in agreement with the observation that there were no significant differences in the plasma clearance rates for these 2 radiolabeled J591 mAb preparations (Table 3). For some target organs (muscle and intestines), the dose estimates for 90Y were significantly higher with 177Lu since the remainder activity was higher with 177Lu compared with that with 111In (Table 4).
Based on clinical studies, Carrasquillo et al. (5) demonstrated the similarities and differences in 111In- and 90Y-labeled mAb distribution. Using DTPA-mAbs, they observed that the differences in the biodistribution between these 2 preparations were between 10% and 15%. The differences in the intravascular kinetics were small, and the major differences were in bone accumulation and urinary excretion of these 2 nuclides.
Since 86Y is a chemically equivalent surrogate for 90Y, Lovqvist et al. (4) recently compared the biodistribution of 86Y- and 111In-labeled mAbs in a nude mouse model. The uptake of these 2 agents at 2 d after injection was generally similar in most tissues. However, after 4 d, 86Y activity was 20%–30% higher in several tissues (liver, spleen, kidney, tumor, bone) compared with that with 111In. In contrast, using DOTA conjugated mAbs, Stein et al. (28) previously reported that 88Y- and 177Lu-labeled DOTA-RS7 mAbs have almost identical biodistribution results in a human lung cancer xenograft model. In a prostate cancer xenograft model, we have recently compared the biodistribution of 111In-, 90Y-, and 177Lu-labeled DOTA-J591 mAbs. We have reported that the biodistribution of 177Lu- and 90Y-J591 were also similar. However, the uptake and retention of 111In activity in the liver and spleen were significantly higher compared with those with either 90Y or 177Lu (29,30). All clinical and preclinical data strongly suggest that the chelating agent used for labeling radiometals to mAbs determines the in vivo stability of radiolabeled mAbs.
CONCLUSION
The trivalent metals, 111In, 90Y, and 177Lu, favor the +3 oxidation state and form strong complexes with the macrocyclic chelator, DOTA. However, there are minor differences in the solution and coordination chemistries among these metals. In patients with prostate cancer, we compared the pharmacokinetics and biodistribution of 111In- and 177Lu-labeled DOTA-J591 mAb. 111In-J591 and 177Lu-J591 have similar plasma and whole-body clearance kinetics. The net retention of 111In activity by lung, liver, and spleen is slightly higher compared with that with 177Lu. Radiation dosimetry estimates for 90Y-J591 calculated based on 111In or 177Lu data were mostly similar and show that the liver is the critical organ, followed by spleen and kidney. These results justify using 111In as a chemical and biologic surrogate for 90Y. In addition, the data also suggest that 177Lu may be a potential alternative for estimating the pharmacokinetics and biodistribution of 90Y-labeled radiopharmaceuticals.
Acknowledgments
I.K. and S.K. were visiting research faculty during 1999–2003 from Kanazawa University, Japan. This work was supported by grants from the U.S. Department of Army (PC970229), the Yablans Research Fund, and the Gerschel Research Fund of the Division of Nuclear Medicine and CaP Cure. N.H.B. is a consultant to BZL Biologics, Inc.
Footnotes
Received Jul. 12, 2004; revision accepted Nov. 17, 2004.
For correspondence or reprints contact: Shankar Vallabhajosula, PhD, New York Weill Cornell Medical Center, 525 E. 68th St., STARR-221, New York, NY 10021.
E-mail: svallabh{at}med.cornell.edu.