Abstract
PET with 18F-FDG has been shown to be useful in the detection and staging of pancreatic cancer. However, whether FDG uptake is dependent on proliferative activity is still unclear. The aim of this prospective study was to evaluate a probable correlation between FDG uptake and proliferative activity in benign and malignant pancreatic tumors. Methods: Our series consisted of 23 patients with pancreatic cancer and 9 patients with chronic active pancreatitis (CAP). FDG PET was performed within 2 wk before surgery, and standardized uptake values (SUVs) were calculated for benign and malignant pancreatic tumors. Patients were selected when focally increased FDG uptake in previously known pancreatic tumors was present. Proliferation fraction was measured in tissue specimens using the anti–Ki-67 antibody MIB-1. A computer-assisted imaging system was used for quantification of nuclear Ki-67 immunostaining. Immunohistochemical findings were correlated to SUVs. Results: Pancreatic cancer showed both intense nuclear staining of Ki-67 (39% ± 16%) and high FDG uptake (SUV = 3.6 ± 1.6). However, no significant correlation was found between in vivo FDG uptake and Ki-67 immunoreactivity (P = 0.65). By contrast, Ki-67 nuclear staining was significantly lower (3.8% ± 2.7%, P < 0.05) in CAP, whereas FDG uptake was in the same range as for pancreatic cancer (SUV = 3.5 ± 1.8). Conclusion: FDG uptake did not correlate with proliferative activity in pancreatic cancer. Proliferative activity was tenfold higher in malignant pancreatic tumors than in benign tumors associated with CAP, whereas FDG uptake in vivo did not differ significantly. Thus, a PET tracer indicating cellular proliferation should better differentiate between cancer and inflammatory lesions than do metabolic markers such as FDG.
The incidence of pancreatic cancer is increasing, and it has the worst prognosis of all types of gastrointestinal cancer. After primary diagnosis, only 20% of patients survive the first year. The mortality ranges from 96% to 99% (1). This poor outcome frequently results from a late diagnosis; the disease has often reached stage III or stage IV—stages commonly associated with distant metastases and peritoneal carcinomatosis (2).
Benign and malignant pancreatic diseases have many gross pathologic findings in common. Enlargement of the pancreatic head, ductal dilatation, cyst formation, infiltration, and ascites can occur with either disease (3). For this reason, morphologic criteria such as the findings of sonography, CT, MRI, or endoscopic retrograde cholangiopancreatography are often less effective for differentiation between malignant and benign pancreatic tumors.
PET using the glucose analog 18F-FDG is a noninvasive imaging technique for tissue characterization based on metabolic differences between benign and malignant tumors. High accuracy in the detection of pancreatic cancer and reliable differentiation between cancer and chronic pancreatitis has been found for FDG PET in several studies, but false-positive findings can occur, especially when acute inflammation is present (4–7). Furthermore, increased expression of glucose transporter-1 gene (glut-1) has been shown in surgically obtained pancreatic cancer specimens (8). However, whether the elevation of glut-1 levels is independent of cancer cell proliferation or reflects a generally increased consumption of substrates associated with proliferating cells has remained unclear. This prospective study was performed to examine whether FDG uptake reflects proliferative activity in human pancreatic tumors.
MATERIALS AND METHODS
Patients
This study consisted of 32 patients (21 men, 11 women; age range, 37–88 y; mean age, 66.2 y). Patients were selected when elevated FDG uptake in previously known pancreatic tumors was present at PET examination. Standardized uptake values (SUVs) were calculated for all patients. All 32 patients had resective pancreatic surgery up to 2 wk after FDG PET. Histopathologic examination of resected specimens indicated malignant disease in 23 (17 ductal adenocarcinomas and 6 ampullary carcinomas) and benign tumors in 9.
The series comprised 8 patients with stage I pancreatic carcinoma, 2 with stage II, 11 with stage III, and 2 with stage IV. All patients with benign tumors had chronic active pancreatitis (CAP). One patient had grade B CAP, 2 patients had grade C, and 6 patients had grade D.
Immunostaining
A standard peroxidase-conjugated streptavidin–biotin complex method (DAKO Diagnostika, Hamburg, Germany) was used; diaminobenzidine (Sigma-Aldrich, Deisenhofen, Germany) served as chromogen. Formalin-fixed, paraffin-embedded sections (5 μm) of resected specimens from pancreatic cancer and chronic pancreatitis were dewaxed and rehydrated and then microwaved in 0.01 mol/L citrate buffer, pH 6.0, for 30 min. For immunostaining, MIB-1 antibody (Dianova, Hamburg, Germany), a monoclonal murine antibody specific for human nuclear antigen Ki-67, was used as the primary antibody in a 1:300 dilution. The sections were lightly counterstained with hematoxylin.
The primary antibody was omitted on sections used as negative controls. Sections obtained from lymph node tissue were included as positive controls for proliferating cells. A highly cellular area of the immunostained sections was evaluated. All epithelial cells with nuclear staining of any intensity were defined as positive. Approximately 600 nuclei were counted on each slide. Proliferative activity was assessed as the percentage of MIB-1–stained nuclei in the sample.
Morphometry
Histopathologic slides were scored by one experienced reader. The fraction of stained nuclei per total nuclei was estimated by counting 600 nuclei per patient using the computer-assisted imaging system OPTIMAS 6.2 (Media Cybernetics, Inc., Silver Spring, MD). Slides were analyzed by light microscopy, and three representative images of each slide were transferred to the computer frame by a video camera connected to the frame-grabber driver. Images were enhanced with various filters to help identify features. The specified measurements were then extracted to the measurement set and exported to export destination (Excel; Microsoft, Redmond, WA) for analysis. Hematoxylin- and eosin-stained fractions were used to estimate the fraction of connective tissue and the fraction of inflammatory cells independently from estimation of the proliferative fraction.
FDG PET
PET was performed using an ECAT 931 08/12 scanner (Siemens Medical Systems, Inc., Hoffman Estates, IL/CTI, Knoxville, TN), which produces 15 contiguous slices per bed position. Axial field of view is 10.3 cm per bed position. Three bed positions were measured for each patient, covering a field of view of 31.5 cm. The emission scan included the liver and the pancreatic region for all patients. Patients fasted for at least 6 h before undergoing PET. Static emission scanning was performed 45 min after injection of 250–300 MBq FDG. The acquisition time was 10 min per bed position. Before FDG administration, 8-min transmission scans with a 68Ge/68Ga ring source were obtained for attenuation correction. Patients were repositioned carefully using laser-guided landmarks to ensure an identical field of view for emission and transmission scanning. Images were reconstructed using an iterative reconstruction algorithm described by Schmidlin (9). The resolution for iterative reconstruction was 7 mm full width at half maximum at the center of the field of view.
Transaxial, coronal, and sagittal sections were documented in a hard copy in a standardized manner. All images were evaluated independently by two experienced readers. Both readers were unaware of the patients’ clinical status. For SUV calculation, circular regions of interest (area, 2 cm3) were drawn around the area with focally increased pancreatic FDG uptake. The regions of interest were approximately two times the minimum resolution of the PET scanner.
Data Analysis
Data are presented as mean, median, and SD. Blood glucose levels, number of Ki-67–positive cells, and FDG SUV of patients with pancreatic cancer and CAP were compared using the Mann-Whitney U test. Differences were considered statistically significant when P was <0.05. Multivariate analysis was used to determine correlations between Ki-67–positive cells, SUV, fibrous tissue, and inflammatory cells in the tumor-surrounding tissue.
RESULTS
FDG PET
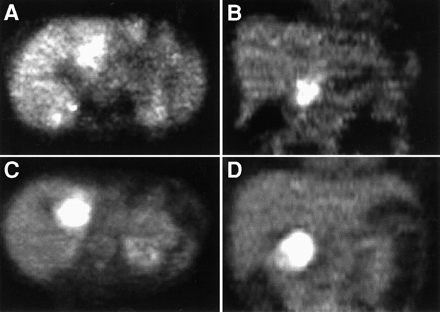
At FDG administration, the mean glucose levels in patients with pancreatic cancer and CAP were 100.5 mg/dL (range, 57–218 mg/dL) and 90 mg/dL (range, 67–135 mg/dL), respectively. The difference was not significant. In ductal adenocarcinoma of the pancreas, the mean SUV was 3.6 ± 1.6 (SD). The median SUV was 4.1, with a range of 1.09–6.78 (Table 1). In CAP, the mean SUV was 3.5 ± 1.8. The median SUV was 4.1, with a range of 1.58–6.42 (Table 2; Fig. 1). FDG PET in patients with CAP revealed an increased FDG uptake mainly in the pancreatic head region, and this increased uptake was similar to that for malignant lesions (Figs. 2A and B). By visual analysis, CAP could not be discriminated from malignant pancreatic disease (Figs. 2C and D).
Data on Patients with Histopathologically Confirmed Pancreatic Carcinoma
Data on Patients with Histopathologically Confirmed CAP
Scattergram of FDG SUVs in patients with histopathologically confirmed ductal adenocarcinoma of pancreas (DPC) and CAP (CP).
(A and B) Iteratively reconstructed FDG PET images (transaxial [A] and coronal [B] slices) of patient with histopathologically confirmed ductal adenocarcinoma of pancreatic head, which shows intense FDG accumulation. (C and D) Corresponding images of patient with histopathologically proven chronic active pancreatitis. Similar focal accumulation of FDG in pancreatic head region is apparent.
Ki-67 Immunohistochemistry
All selected specimens of pancreatic cancer tissue contained Ki-67–positive cells. Stained nuclei belonged mainly to epithelial cells; a very small portion belonged to inflammatory cells. Ki-67 positivity ranged from 6.5% to 62.0% of sampled epithelial nucleus profiles (median value, 35%) (Table 1). The mean fraction of Ki-67–positive nuclei was 38.5% (SD, 15.5%). In 11 patients, >40% of nuclei showed immunoreactivity for Ki-67 antigen (Fig. 3).
Typical cross section of pancreatic adenocarcinoma stained with antihuman nuclear Ki-67 antibody MIB-1 and lightly counterstained with hematoxylin. Amount of Ki-67–positive tumor cells is approximately 60% in this cross section. (×200)
Ki-67–positive cells were present in eight cases of CAP. One tissue specimen did not show any immunoreactivity to Ki-67 antigen. The range for Ki-67–positive cells was 0%–7.0% (Table 2; Fig. 4). Ki-67–positive nuclei belonged mainly to inflammatory cells rather than to epithelial cells. The mean of Ki-67–positive cells in CAP was 3.8% (SD, 2.7%). In pancreatic cancer, compared with CAP, significantly more Ki-67–positive nuclei could be evaluated (P < 0.05; Fig. 5).
Cross section of CAP stained with antihuman nuclear Ki-67 antibody MIB-1 and lightly counterstained with hematoxylin. Amount of Ki-67–positive tumor cells is approximately 2% in this cross section. Left upper part of figure shows newly formed reactive lymph node with lightly positive Ki-67 immunostaining. (×200)
Scattergram of Ki-67 values in patients with histopathologically confirmed ductal adenocarcinoma of pancreas (DPC) and CAP (CP). pos. = positive.
Regional lymph nodes serving as positive controls showed an intense nuclear staining with Ki-67 antibody. In control sections, in which the primary antibody was omitted, no positive nuclear staining was visible.
Inflammatory cells were detected in the tumor-surrounding tissue in 21 pancreatic cancer specimens. No inflammatory cells were present in 2 specimens. The mean fraction of inflammatory cells was 15%, and SD was 8.6%. In two patients with CAP, no inflammatory cells could be detected. The mean percentage of inflammatory cells in the tumor-surrounding tissue for benign lesions was 12.2%, and SD was 9.7%.
Approximately 50% of the tumor tissue within the cancer specimens consisted of stroma (SD, 16.9%). Fibrous tissue was present in all sections (range, 20%–80%). In benign lesions, the mean volume fraction of fibrous tissue was 35% (SD, 20.7%). The minimum percentage was 10%, and the maximum was 60%.
Correlation Between Proliferative Activity and FDG Uptake
Intense immunoreactivity to Ki-67 antigen was present in pancreatic carcinomas with strongly increased FDG uptake as well as in those with low FDG uptake (Table 1). No significant correlation was found between Ki-67 immunoreactivity and FDG uptake (P = 0.65). Statistical analysis also showed no significant correlation between FDG uptake and the presence of inflammatory cells in tissue specimens (P = 0.47).
The low immunoreactivity to Ki-67 antigen in CAP suggested a possible correlation with focally increased FDG accumulation in the pancreas (P = 0.09; Table 2). No correlation was found between FDG uptake and the number of inflammatory cells (P = 0.44).
In pancreatic cancer, multivariate analysis showed a possible correlation between the number of fibrocytes and SUV (P = 0.06). In CAP, multivariate analysis showed no correlation between the number of fibrocytes and SUV (P = 0.48).
DISCUSSION
Using morphologic imaging modalities such as sonography and CT, differentiation of pancreatic adenocarcinoma from benign pancreatic tumors associated with CAP often remains difficult. Functional imaging with FDG PET has been shown to be more accurate than these modalities but less specific when acute inflammation is present (10,11).
Expression of glut-1 and membrane glucose transport is generally increased in pancreatic cancer but not in chronic pancreatitis (8). On the other hand, avid FDG uptake caused by increased glycolytic activity has been shown in inflammatory cells such as neutrophils and activated macrophages, which are present in areas of acute or chronic inflammation (12,13). Accordingly, FDG has been reported to accumulate in various inflammatory processes (14,15), including acute pancreatitis (16). Nevertheless, Kato et al. (17) showed a significantly higher FDG uptake in patients with pancreatic cancer than in those with mass-forming pancreatitis. False-positive findings were observed, however, in two of nine patients with scar tissue associated with leukocytic infiltration. Similar results have been reported for a larger series (11,18). To minimize false-positive PET findings, Diederichs et al. (11) recommended, therefore, that patients be excluded if their laboratory data show evidence of acute bouts of chronic pancreatitis.
Pancreatic FDG PET, being less sensitive for cancer detection in patients with elevated plasma glucose levels (6,19), is also affected by other metabolic conditions such as altered glucose metabolism. In our series, blood glucose level was >130 mg/dL in three patients with pancreatic cancer. FDG uptake might therefore be reduced in these patients.
Our results applied to a particular selection of patients with focally increased FDG uptake in the pancreas. Therefore, the accuracy of FDG PET in detecting pancreatic malignancies could not be evaluated. FDG uptake was similarly increased in CAP and in pancreatic adenocarcinoma (Fig. 1; Tables 1 and 2). Moreover, the pattern of FDG accumulation in CAP could not be visually discriminated from that in malignant lesions (Fig. 2). Our results indicate that FDG PET is of limited value for differentiating between pancreatic cancer and pancreatitis in patients with acute episodes of chronic pancreatitis. Differentiation between active inflammation and malignant tumors with FDG PET therefore remains problematic.
Our most important finding was that Ki-67 immunoreactivity enabled reliable differentiation between benign and malignant pancreatic tumors (Fig. 5). Ki-67 is a nuclear antigen expressed in the G1, G2, and S phases of the cell cycle but not in the G0 phase (20). Currently, Ki-67 is therefore an accepted marker for proliferative activity (21,22). In our study, the mean percentage of Ki-67–positive cells was approximately tenfold higher in pancreatic cancer than in CAP, indicating that proliferative activity is elevated strongly in the former but only slightly in the latter. For pancreatic tumors, radiotracers such as 11C-thymidine or 18F-3′-deoxy-3′-fluorothymidine, which indicate proliferative activity (23), should be more suitable for differentiating between malignancies and inflammatory processes than are metabolic markers such as FDG.
Data on the correlation between proliferative activity and glycolysis in malignant tumors, as measured by FDG uptake, are controversial. Okada et al. (24) found a positive correlation between proliferation and FDG uptake in a small series of malignant lymphoma cases. By contrast, the in vitro results of Higashi et al. (25) indicated no correlation between proliferative activity and FDG uptake in human cancer cells. In agreement with this in vitro study, our pancreatic carcinoma study revealed no correlation between FDG uptake and proliferative activity.
CONCLUSION
Our results showed no correlation between proliferative activity and FDG uptake in pancreatic cancer. Overexpression of the glut-1 gene as measured by increased FDG uptake, and proliferation as measured by Ki-67, seem to be independent processes in pancreatic cancer. In consideration of similar FDG uptake values but a tenfold higher proliferative activity for pancreatic cancer than for CAP, differentiation of pancreatic cancer from CAP should be feasible with radiotracers that indicate proliferative activity rather than with metabolic tracers such as FDG.
Acknowledgments
This study was supported in part by research grant 516 from Deutsche Forschungsgemeinschaft.
Footnotes
Received Aug. 22, 2000; revision accepted Dec. 11, 2000.
For correspondence or reprints contact: Andreas C. Buck, MD, Department of Nuclear Medicine, University of Ulm, Robert-Koch-Strasse 8, D-89070 Ulm, Germany.