Abstract
We report improved incorporation of the radiolabeled-thymidine analog [125I/131I]5-iodo-2′-deoxyuridine ([125I/131I]IdUrd) into DNA by the addition of Thymitaq, a thymidylate synthase inhibitor, as a strategy of molecular radiotherapy for hepatoma treatment. Methods: The synergistic effect of combination [125I]IdUrd and Thymitaq in clonogenic survival and DNA incorporation was shown on the human hepatoma cell line Hep3B. Radiobiodistribution of intrahepatic arterially injected [125]IdUrd and Thymitaq was studied in a rat N1S1 hepatoma model. In vivo therapeutic effects of locoregional delivery of both drugs were evaluated in mouse subcutaneous hepatoma and ascitic hepatoma models. Results: In a clonogenic assay, Thymitaq showed a synergistic effect with [125I]IdUrd but not cold IdUrd. Thymitaq had a dose-dependent modulation effect on DNA-[125I]IdUrd incorporation. The biodistribution study indicated a slower clearance rate of [125I]IdUdR in the hepatoma as well as an initially higher uptake of [125I]IdUrd into DNA when the [125I]IdUrd was combined with Thymitaq. In vivo studies showed a superior therapeutic effect of combination Thymitaq and [125I]IdUrd in both subcutaneous and ascites tumor models, but the combination of [131I]IdUrd and [125I]IdUrd may be more effective than Auger electron emitters alone for the treatment of subcutaneous tumor. Conclusion: The strategy of locoregional delivery of [125I/131I]IdUrd to a tumor site through an intrahepatic arterial, intratumoral, or intraperitoneal route in combination with Thymitaq is promising and may also have a favorable therapeutic index in vivo.
Iododeoxyuridine (IdUrd) is a halogenated thymidine analog that competes with thymidine in the biosynthesis of DNA. IdUrd has long been regarded as a potent radiosensitizer, but clinical use is limited because of its low incorporation ratio during conventional administration schedules, as well as its low cytotoxicity (1,2). Because IdUrd competes with thymidine for DNA incorporation, the size of the thymidine pools, that is, the amount of extracellular and intracellular thymidine, is critical to the percentage of IdUrd incorporated into DNA (3,4). Administration of a thymidylate synthase (TS) inhibitor (TSI) inhibits the de novo pathway of thymidine monophosphate (dTMP) synthesis. Less intracellular thymidine is then available to compete with IdUrd; therefore, the percentage of IdUrd incorporation into DNA is increased.
Although the in vitro modulation effect of TSI on IdUrd incorporation has been reported by several investigators (5–7), the systemic administration of IdUrd plus TSI does not translate into better tumor control in vivo. To increase the killing efficiency of IdUrd, this nucleotide can be labeled with an isotope and used as a carrier to incorporate isotopes into DNA. IdUrd labeled with isotope has great potential as a radiotherapeutic agent, because it is capable of targeting dividing cells. Iodine-125 decay emits highly radiotoxic Auger electrons, which have an ultra-short range but high linear energy transfer in matter. Targeting 125I into DNA, which has an effective range of only a few nanometers, results in more than one double-strand break per decaying atom (8).
This cell cycle–specific molecular radiotherapy strategy is especially attractive in hepatoma treatment, because normal surrounding tissues are relatively quiescent. However, a proportion of malignant cells are not in the DNA-synthetic phase of the cell cycle during the time of exposure to [125I]IdUrd, which greatly limits its clinical application. Repeated or prolonged administration of [125I]IdUrd may partially overcome this problem. Another potential complementary approach is the combination of [131I]IdUrd and [125I]IdUrd. The β-emitter form of [131I]IdUrd, although less radiotoxic to cells than Auger electrons, produces relatively longer-range β electrons, resulting in the irradiation of neighboring nonproliferating cells by a cross-fire effect (9,10). We now report the modulating effect of TSI on IdUrd-DNA incorporation and the synergistic cytotoxic effect of the combination of TSI and [125I]IdUrd on Hep3B monolayer cells. The antitumor effect of the combination of TSI and the locoregional delivery of radiolabeled IdUrd was evaluated both by intratumoral administration in mouse models of subcutaneous hepatoma and by intraperitoneal administration in mouse models of ascites hepatoma. Finally, we evaluated the radiobiodistribution of radiolabeled IdUrd by the intra-arterial administration route in a rat hepatoma model.
MATERIALS AND METHODS
Chemicals
Thymitaq AG337 was a gift from Agouron Pharmaceuticals (San Diego, CA). IdUrd was purchased from Sigma (St. Louis, MO). 5-[125I/131I]IdUrd was synthesized, as described previously, with some modifications (11). Briefly, 100 μL of oxidizing agent (H2O2/1N HCl/H2O = 4:1:95) was added to a 300-μL v-vial coated with 50 μg (0.1 μmol/L) 5-tributylstannyl-2′-deoxyuridine containing 20 μL ethanol and 3.7–37 MBq (∼0.1–1 mCi) sodium [125I/131I]iodide. The reaction mixture was set aside and vortexed intermittently. After 8 min, the mixture was frozen in liquid nitrogen, then lyophilized under vacuum for ∼1 h to give the final product as a “hot kit.” Unreacted [125I/131I]iodide (in the form of I2 in the presence of an oxidizing agent), HCl, solvents (ethanol and H2O), and oxidizing agent (H2O2) were removed during lyophilization. The lyophilized [125I/131I]IdUrd hot kit was redissolved in ethanol, and the radiochemical purity was determined using thin-layer chromatography (TLC) and high-pressure liquid chromatography (HPLC). TLC was performed on a TLC aluminum sheet (Silica gel 60 F254; Merck, West Point, PA), using ethyl acetate/ethanol (85:15 [vol/vol]) as the mobile phase. Chromatograms were recorded using an imaging scanner (system 200; Bioscan). The Rf value of [125I/131I]IdUrd was ∼0.62–0.64. HPLC analysis was performed on a reversed-phase column (RPR-1; Hamilton) using methanol/water (20:80 [vol/vol]) as the eluant at a flow rate of 1 mL/min. An ultraviolet (UV) detector (Tunable absorbance detector 486; Waters, Milford, MA) and a radiodetector (Capintec; Bioscan) were used to analyze the eluates. Data were collected and analyzed with computer software (CSW, version 1.7; DataApex Ltd., Prague, Czechoslovakia). The retention time of [125I/131I]IdUrd was ∼7.2–7.4 min. The labeling yield was >95%, and the radiochemical purity was >98%. The lyophilized [125I]IdUrd product was stable for up to 3 wk. The lyophilized [125I/131I]IdUrd hot kit, if dissolved in physiologic saline and eluted through a 0.22-μm apyrogenic disk, was ready for biologic or clinical application.
Incorporation of [125I]IdUrd into Nucleic Acid in Hep3B Cells
To assess the effect of Thymitaq on the incorporation of [125I]IdUrd into nucleic acids, exponentially growing cells were exposed to [125I]IdUrd at 0.37 MBq/mL (10 nmol/L) for the last 3 h of a 5-h Thymitaq exposure (2–10 μmol/L). The cells were harvested, and the remaining isotopes were washed three times with phosphate-buffered saline (PBS) and pelleted. The cells were extracted twice with 100 μL 0.2N perchloric acid. The insoluble material was incubated at 37°C for 20 min with 100 μL RNase solution (7.5 mg DNase-free RNase plus 50 mL 50 μmol/L Tris-ethylenediaminetetraacetic acid [EDTA], pH 7.4). The reaction was terminated by the addition of 200 μL 0.2N perchloric acid and centrifuged at 17,500g for 10 min. The pellet was then counted in an IS-330 Beckman scintillation counter (Fullerton, CA).
Cell Cultures and Clonogenic Assay
Human hepatoma cell line Hep3B cells were maintained as monolayers in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS). The doubling time of the cells was ∼24 h, and the plating efficiency was 70%. Cells were incubated in 2 μmol/L Thymitaq–10 μmol/L IdUrd with or without adding 0.37 or 0.74 MBq or [125I]IdUrd for different time intervals. The cells were then trypsinized, resuspended, counted, and serially diluted in fresh medium, and an appropriate number of cells was plated on 60-mm-diameter culture dishes (Corning Inc., Corning, NY). The dishes were incubated for an additional 8–9 d at 37°C. Cells were then fixed and stained with 0.5% crystal violet in methanol/acetic acid (3:1 [vol/vol]). Colonies having >50 cells were scored.
In Vivo Therapy Studies
Cells and Tumors.
The C3H inbred murine hepatoma model, MH134, is an ascites hepatoma cell. The cell line 1MEA 0.7R is a hepatoma cell line acquired from inbred BALB/C mice.
Subcutaneous Tumor Model.
The 1MEA 0.7R cells were harvested by trypsinization, suspended in DMEM supplemented with 10% FCS, centrifuged at 250g for 10 min, and resuspended in culture media at a concentration of 5 × 106 cells/mL before subcutaneous implantation into mice. Female BALB/c mice, 4–6 wk old, were implanted with 5 × 105 cells into the right flank with a 27-gauge needle in a 1-mL tuberculin syringe. Approximately 10 d later, when the tumor reached ∼4 × 4 mm in diameter, mice were randomly assigned to groups, including control, radiolabeled IdUrd, Thymitaq, and different combinations. Thymitaq (135 mg/kg) was given by intraperitoneal injection, [125I]IdUrd or [131I]IdUrd was delivered by intratumoral injection at a dose of 3.7 MBq in 6% gelatin. The combined [125I/131I]IdUrd was injected as a mixture of 1.85 MBq of each isotope intratumorally in 6% gelatin for a total volume of 100 μL. The control and Thymitaq-alone groups were also intratumorally injected with 6% gelatin in a 100-μL volume. Each group had six mice in each experiment, and two experiments were performed. Tumors were measured using calipers, and tumor volumes were calculated (tumor volume = length × width2/2) every 2–3 d. Mice were killed when the tumor ulcerated or reached a diameter of more than 2 cm.
Ascites Tumor Model.
After MH134 cells were collected from the ascites fluid from tumor-bearing mice, these cells were then suspended in DMEM at a concentration of 1 × 106 cells/mL before intraperitoneal implantation into inbred C3H mice. Female C3H mice, 4–6 wk old, were implanted with 5 × 105 cells using a 26-gauge needle in a 1-mL tuberculin syringe. Mice were randomly assigned to four groups: the control group, mice receiving Thymitaq (45 mg/kg) intraperitoneally four times daily × 3 d, mice receiving [125I]IdUrd (1.23 MBq in 1 mL) intraperitoneally four times daily × 3 d, and mice receiving a combination of two drugs on day 4 after tumor inoculation. Each group consisted of five mice for each experiment, and two experiments were performed. Survival time was recorded for each mouse and plotted by the Kaplan–Meier method (12).
Rat Hepatoma Model.
Male Sprague-Dawley (SD) rats weighing ∼250 g were used in this study. An N1S1 hepatoma cell line, which originated from an SD rat, was cultured in vitro before transplantation into the liver. Generally, the N1S1 tumor cells were cultured in DMEM mixed with 5% FBS, 20% horse serum, and 1% l-glutamine. Before tumor cell inoculation, the rats were anesthetized by intraperitoneal injection of ketamine at a dose of 10 mg/100 g of body weight. Subxyphoid laparotomy was performed to expose the left lobe of the liver for inoculation of tumor cells. A microsyringe with a 27-gauge needle was used to inject 4 × 106 cells/0.1 mL into the left hepatic lobe. Laparotomy was again performed ∼2 wk after inoculation to check tumor growth.
Hepatic Artery Ligation and Cannulation.
In brief, a small arteriotomy was made into the hepatic artery, and the tip of PE-10 tubing (Becton Dickinson, Annapolis, MD) was inserted into the artery. The catheter was then secured in place by a ligature placed around the artery. The implanted port was secured in place by suturing it to the underlying muscular fascia, and the port pocket was closed. Finally, the hepatic artery infusion was performed by percutaneous injection of the agents into the port chamber as required. The rats were divided into two groups for [125I]IdUrd radiobiodistribution studies: group 1, with Thymitaq pretreatment; and group 2, without Thymitaq pretreatment. At each of three time points (4, 12, and 24 h after infusion), three rats were studied. In each rat, Thymitaq (135 mg/kg) was given intra-arterially through the port in 1 min, and [125I]IdUrd was injected 2 h later in a dose of 3.7 MBq through the port in 1 min. Normal saline (0.5 mL) was flushed into the port after each injection.
Biodistribution of IdUrd in Hepatoma and Normal Tissues by γ-Counting
The rats were killed with sodium pentobarbital at 4, 12, and 24 h after injection of 3.7 MBq [125I]IdUrd through the hepatic artery. Samples of hepatoma, liver, and large intestine were taken and cut into pieces (about 0.1 g/piece) and weighed. Two milliliters of enzyme cocktail solubilizer were added to each sample and incubated at 50oC for 3 h to achieve tissue lysis. After complete lysis of the tissue, equal volumes of lysed tissue were transferred for γ-counting. Finally, the tissue concentrations of [125I]IdUrd were expressed as counts per minute per milligram of tissue.
RESULTS
Figure 1 shows a linear dose-dependent increase in [125I]IdUrd incorporation into DNA fractions of Hep3B cells with increased doses of Thymitaq. We noticed a sevenfold increase in 125I radioactivity after 5 h of exposure to Thymitaq, which indicates that Thymitaq is an effective modulator for increasing incorporation of IdUrd into DNA.
Increase in [125I]IdUrd incorporation into DNA in presence of Thymitaq relative to [125I]IdUrd alone in Hep3B cells. Cells were harvested after incubation in 370 kBq [125I]IdUrd for last 3 h of 5-h exposure to Thymitaq at different concentrations (2–10 μmol/L). DNA was extracted by PCA precipitation, and pellets were counted for radioactivity.
Clonogenic survival was determined in Hep3B cells exposed to 2 μmol Thymitaq/L and 10 μmol IdUrd/L with or without 0.37 or 0.74 MBq [125I]IdUrd for different time intervals. Results from Figure 2 indicate that the combination of Thymitaq and IdUrd has only very limited additive effect, but the addition of 0.37 MBq [125I]IdUrd/mL (10 nmol/L) results in synergy, even though the radiolabeled IdUrd added is at a concentration of <1/1,000 of its surrounding milieu (10 μmol/L cold IdUrd). A strong synergistic antitumor effect of Thymitaq and radiolabeled IdUrd in hepatoma cells could be observed.
Clonogenic survival of Hep3B cells exposed to 2 μmol/L Thymitaq or 10 μmol/L IdUrd with or without 0.37 or 0.74 MBq [125I]IdUrd for different time intervals. Treatment groups were divided as follows: ○, Thymitaq (2 μmol/L); •, IdUrd (10 μmol/L); □, Thymitaq (2 μmol/L) + IdUrd (10 μmol/L); ▪, IdUrd (10 μmol/L) + 125IdUrd (0.37 MBq); ▵, IdUrd (10 μmol/L + 125IdUrd (0.74 MBq); ▴, Thymitaq (2 μmol/L) + IdUrd (10 μmol/L + 125IdUrd (0.37 MBq); ▾, Thymitaq (2 μmol/L) + IdUrd (10 μmol/L) + 125IdUrd (0.74 MBq).
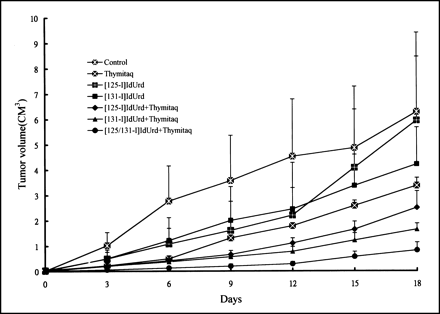
[125I]IdUrd was also effective therapeutically against a subcutaneously inoculated mouse 1MEA 0.7R hepatoma model when intratumoral injection was used. As shown in Figure 3, a moderate delay in tumor growth was observed by the administration of intraperitoneal Thymitaq and a slight growth delay by intratumoral [125I]IdUrd. A better therapeutic effect was achieved by the administration of both Thymitaq and [125I]IdUrd or [131I]IdUrd. The antitumor effect was most significant when [131I]IdUrd and [125I]IdUrd were administered together intratumorally, along with Thymitaq administered intraperitoneally. Figure 3 shows the additional response resulting from the cross-firing effect of 131I and 131I.
Effect of Thymitaq, [125I]IdUrd, [131I]IdUrd, and different combinations on tumor growth. 1MEA 0.7R tumor-bearing mice were injected with Thymitaq (135 mg/kg intraperitoneally); intratumoral injection of 3.7 MBq [125I]IdUrd or [131I]IdUrd in 6% gelatin in 100-μL volume; intratumoral injection of combination of mixture of 1.85 MBq of each isotope [125I/131I]IdUrd in 6% gelatin in 100-μL volume. Tumors were measured every third day. Each group had six mice in each experiment, and two experiments were performed. All data shown are mean ±1 SD.
Figure 4 shows the overall survival of MH134-ascites-bearing mice after [125I]IdUrd treatment. The intraperitoneal inoculation of MH134 ascitic hepatoma cells resulted in 100% mortality of C3H mice, with 20-d median survival. The intraperitoneal administration of [125I]IdUrd or Thymitaq had no effect on median survival, whereas Thymitaq prolonged the median survival time to 26 d. The combination of Thymitaq and [125I]IdUrd treatment further prolonged the median survival time to 33 d and, possibly more important, resulted in a 20% cure (2 of 10 mice).
Effect of Thymitaq, [125I]IdUrd, or combination on overall survival of ascites-bearing mice. MH134 cells were collected from ascites fluid from tumor-bearing C3H mice. Mice were inoculated intraperitoneally with 5 × 105 cells. Mice were randomly assigned to four groups of five mice: control, Thymitaq (45 mg/kg intraperitoneally four times daily for 3 d), [125I]IdUrd (1.3 MBq intraperitoneally four times daily for 3 d), or combination of two drugs on day 4 after tumor inoculation.
Figure 5 shows the biodistribution of [125I]IdUrd in N1S1-hepatoma-bearing SD rats determined at 4, 12, and 24 h after intra-arterial injection of 3.7 MBq/0.1 mL radioactive IdUrd with or without Thymitaq injection. An initial 1.6-fold-higher tumor retention dose was found 4 h after injection in the Thymitaq pretreatment group than that found in the group receiving [125I]IdUrd alone, and it was 8.4-fold higher 24 h after injection (P < 0.01). The retention ratios between hepatoma and normal hepatic tissue (T/Ls) were 1.43 at 4 h, 2.18 at 12 h, and 1.5 at 24 h after [125I]IdUrd injection. The T/Ls were 2.26 at 4 h, 2.23 at 12 h, and 2.62 at 24 h in the Thymitaq pretreatment group. The tumor retention dose between 4 and 24 h was 32% in the Thymitaq treatment group, whereas it was 6% in the group receiving [125I]IdUrd alone (P < 0.05). The findings indicate that there is a slower clearance of [125I]IdUdR from the tumors in the Thymitaq treatment group. The slower clearance plus an initial higher uptake of [125I]IdUrd into DNA by Thymitaq would be expected to increase the area under the curve of total isotope retention and allow enough decay of [125I]IdUrd to kill the cells.
Radiobiodistribution of [125I]IdUrd in tumor, normal liver, and large intestine at different time intervals with or without Thymitaq. Port-implanted hepatoma-bearing rats were divided into two groups, with (A) or without (B) Thymitaq pretreatment. There were three time points (4, 12, and 24 h), and each time point had three rats. Thymitaq was injected over 1 min at 135 mg/kg intra-arterially through the port, and 2 h later [125I]IdUrd was injected over 1 min at 3.7 MBq through the port. Tissue concentrations of [125I]IdUrd are expressed as counts per minute (cpm) per milligram of tissue.
DISCUSSION
This study assessed the modulating effect of TSI on IdUrd incorporation into DNA and also tested the hypothesis that TSI and radiolabeled IdUrd are effective in an animal model, which could be extended to a clinical strategy. Because of the short half-life and poor tumor-targeting effect of IdUrd, this drug may be better used by locoregional administration than by systemic injection. The model chosen for the present investigations was hepatoma. Hepatoma is an ideal therapeutic model for molecular radiotherapy because of the feasibility of intra-arterial injection of radiolabeled IdUrd through the hepatic artery and the relatively quiescent surrounding liver tissue. We first established a rat hepatoma tumor model and performed intra-arterial cannulation for the study of radiobiodistribution of [125I]IdUrd in the rat. We could not exclude the possibility that part of the response seen in the N1S1 rat model may have been mediated by immunologic rejection. Therefore, we investigated the therapeutic effect of radiolabeled IdUrd in other locoregional tumor models, such as C3H ascites and BALB/c subcutaneous-hepatoma models.
The synergistic effects of combination IdUrd and TSI have been reported previously in vitro and in vivo (5–7). The mechanism was postulated to result from decreased TS activity and therefore to a decrease in the deoxythymidine triphosphate pools that could compete with iododeoxyuridine triphosphate as a substrate for DNA polymerase (13,14). The inhibition of TS would also decrease the dehalogenation of iododeoxyuridine monophosphate by TS and increase the activity of thymidine kinase and thymidylate kinase (15,16). One explanation for the slower clearance of IdUrd could be that the metabolic dehalogenation of [125I]IdUrd by TS was inhibited. Another possibility could be that there is an active equilibrium between IdUrd incorporated in DNA that ultimately exchanges over time with thymidine. Our in vitro data do not exclude either possibility. We were able to show a synergistic interaction of cold IdUrd and TSI. The importance of prolonged exposure with another TSI, D1694, for maximal TS inhibition was shown previously (4). However, in the current study, we do not find a synergistic effect from a combination of these two drugs when monitored for up to 24 h of exposure in hepatoma cells. On the contrary, a synergistic effect between TSI and radiolabeled IdUrd was observed after only a few hours of exposure. Our results suggest that prolonged inhibition of TSI is not necessary to achieve a synergistic effect on the radiocytotoxicity of [125I]IdUrd. A short exposure (2–5 h) with Thymitaq and radiolabeled IdUrd in our in vitro and in vivo models has resulted in a therapeutic gain. Longer exposure of Thymitaq and IdUrd would be expected to increase the risk of simultaneous sensitization of both normal and tumor tissues and therefore to produce a loss of therapeutic benefit. Shorter but more frequent administration of both agents would appear to catch a greater proportion of S-phase cells in the tumor and minimize the uptake of cycling normal tissues (17,18). We are currently advancing further studies to determine the optimal schedule to achieve the best therapeutic index.
Mester et al. (7) reported that the systemic delivery of [125I]IdUrd and TSI leads to only a limited absolute increase in the uptake of [125I]IdUrd by tumors, despite a relatively lowfold increase in delivered radioactivity. Their results do not support the clinical strategy of systemic administration of [125I]IdUrd. However, the insufficient incorporation of the radioactive DNA precursor may be surmounted by locoregional delivery. Locoregional delivery has circumvented some of the limitations associated with systemic delivery of intact [125I]IdUrd. Preliminary trials with intratumoral injection and intra-arterial injection of [125I]IdUrd in patients with liver metastases were reported by Mariani et al. (19,20). They found excellent targeting of [125I]IdUrd to a tumor in the central nervous system, as well as in a local breast cancer model (21,22). The high retention rate found by using this strategy suggested that the radiation dose can be sufficiently enhanced by the combination of TSI and [125I]IdUrd. A few-fold increase in the radioactivity from this combination would then create a favorable chance for tumor control. Our results, as well as similar observations from Bagshawe et al. (6), suggest that not only the higher the dose of [125I]IdUrd but also the higher the dose of TSI will increase the IdUrd incorportion rate (6). Locoregional delivery of both agents would be expected to achieve the highest concentration possible without untoward effects seen during systemic administration.
The success of curative targeting strategies is governed by the ability to sterilize all clonogens. The amount of radioactive precursor incorporated into tumor cells in vivo is several orders of magnitude lower than is seen when using cell culture conditions. The lack of notable antitumor activity of [125I]IdUrd in vivo is caused by insufficient incorporation of the radioactive DNA precursor. The uptake of [125I]IdUrd is unavoidably limited by the presence of noncycling and slowly cycling cells. Therefore, one strategy to overcome the problems would be the use of a slow-release drug delivery system by incorporating radiolabeled IdUrd in 6% gelatin. The combination of β-emitters and Auger electrons to kill the cells would be expected to provide extra tumor control. Slow release of DNA precursors from gelatin would be expected to increase the chance of radioactivity uptake by slowing cycling cells (23). The combination of short- and medium-range emitters would provide immediate kill and targeting of adjacent cells that are not cycling (10).
Neshasteh-Riz et al. (10) in their spheroid model reported that both [125I]IdUrd and [123I]IdUrd showed a strong dose-dependent effect at low concentrations, but their cytotoxicity reached a maximum of ∼55%–70% and did not further increase, even at activity concentrations of >40 KBq mL−1. In contrast to the Auger electron emitters, [131I]IdUrd was progressively more toxic at increasing doses in the spheroid model (10). Auger electron emitters located on the cell membrane or in the cytoplasm are relatively nontoxic, whereas DNA-incorporated Auger electrons are highly radiotoxic. On the contrary, β-emitters located in the DNA, cell membrane, or cytoplasm can be cytotoxic. The absorbed fraction of the decay energy of 131I would scatter electrons into adjacent noncycling cells, whereas cycling cells would be killed by Auger electrons.
As noted earlier, slowly proliferating cells were the most difficult to eradicate. Slow-release IdUrd from polymeric microspheres was previously developed for different purposes (24,25). In an attempt to prolong the absorption of IdUrd, we used 6% gelatin solution as the delivery vehicle in this experiment. Hampton et al. (23) recently reported a 1.5-fold increase in 131I activity in tumor tissue after intramuscular injection of [131I]IdUrd in 16% gelatin. Use of oil emulsion increased by a factor of 3 uptake of the 131I content of wet tumor tissue in the same experiment (23). We are now attempting to use lipiodol as a carrier for targeting [125I/131I]IdUrd into hepatoma by intra-arterial injection. Intra-arterial 131I-Lipiodol has been used as adjuvant therapy for patients who have undergone surgical resections as well as primary therapy for patients with inoperable hepatoma, and both are reported to be well tolerated and effective (26,27). We have shown a promising radioactivity retention rate by intra-arterial injection of [125I]IdUrd-Lipiodol in the rat model (28).
CONCLUSION
We have shown that the maximum therapeutic benefit of radiolabeled IdUrd molecular radiotherapy may be derived from using TSI and both Auger electron emitters to kill cycling cells and β-emitters to eliminate noncycling cells. We are now preparing a phase I trial of intra-arterial injection of [125I/131I]IdUrd, which will use lipiodol as the carrier vehicle for the treatment of advanced hepatoma.
Acknowledgments
The authors thank Jin-Yee Chao, MSc, for statistical assistance and also thank Chue-Kwei Lin for excellent technical assistance. The study was supported by grant 88-403 from Veterans General Hospital, Taipei, Taiwan, Republic of China.
Footnotes
Received May 10, 2000; revision accepted Aug. 28, 2000.
For correspondence or reprints, contact: Kwan-Hwa Chi, MD, Cancer Center, Veterans General Hospital-Taipei, #201, Sec 2, Shih-Pai Rd., Taipei 11217, Taiwan, Republic of China.