Abstract
In vitro and in vivo experiments from our laboratory and others have suggested that the combination of thymidylate synthase (TS) inhibitor and radiolabeled iododeoxyuridine (IdUrd) is synergistic. Efficacy is limited by drug resistance, which is often mediated by TS overexpression. We designed an in vivo electrogene transfer (EGT) model for delivering antisense TS plasmid (ATS) into tumor to increase the subsequent efficacy of 131I-IdUrd therapy. Methods: Plasmid complementary to nucleotide 531-710 in the coding region of the mouse TS (mTS) mRNA was constructed. TS activity and 131I-IdUrd DNA incorporation were determined 48 h after in vitro EGT of ATS to CT26 cells. In vivo therapeutic effect and radioactivity retained in tumor after various combinations of EGT ATS, 5-fluorouracil (5-FU), and continuous infusion of 131I-IdUrd by osmotic minipump were determined. Results: A reduction of TS activity was achieved after in vitro EGT ATS. Flow cytometry analysis indicated that ATS-treated cells were arrested at S phase. In the in vivo tumor model, the combination of EGT ATS and 5-FU was able to partially overcome 5-FU drug resistance. Sixty percent of tumors can be eradicated by the combination of EGT ATS, 5-FU, and infusion of 131I-IdUrd. The tumors treated by EGT ATS had higher radioactivity retained 1 wk after 131I-IdUrd therapy than after EGT of control plasmid. Conclusion: In situ EGT ATS can downregulate TS and increase the therapeutic effect of radiolabeled IdUrd therapy. The combination of EGT ATS, 5-FU, and 131I-IdUrd may result in tumor eradication.
- 5-fluorouracil
- antisense
- electrogene transfer
- radiolabeled iododeoxyuridine
- thymidylate synthase inhibitor
Iododeoxyuridine (IdUrd) is a halogenated thymidine analog that competes with thymidine in the biosynthesis of DNA. Cytotoxicity is dependent on its cellular uptake, activation, and subsequent incorporation into DNA (1,2). IdUrd has long been regarded as a potent radiosensitizer, but clinical use is limited because of low IdUrd incorporation. IdUrd labeled with radioisotopes has great potential as a radiotherapeutic agent, because it is capable of targeting dividing cells and emitting highly radiotoxic Auger electrons from 125I-IdUrd inside DNA and β-rays from 131I-IdUrd (3,4). We have demonstrated that if just a few nanomoles per liter of IdUrd are replaced as 125I-IdUrd, the killing efficiency can increase more than 2 log (5). Because IdUrd competes with thymidine for DNA incorporation, the size of thymidine pools is critical to the IdUrd DNA incorporation ratio. Administration of thymidylate synthase (TS) inhibitor inhibits the de novo pathway of thymidine synthesis and decreases intracellular thymidine available to compete with IdUrd and, therefore, increases the IdUrd DNA incorporation. The use of TS inhibitor to increase the radiolabeled IdUrd uptake has been reported by several investigators (5,6). The higher the TS inhibition, the higher would be the radiation dose.
The active conversion of 5-fluorouracil (5-FU), 5-fluorodeoxyuridine monophosphate (FdUMP), is a specific TS inhibitor. Changes in TS level or its affinity for FdUMP have been associated with 5-FU resistance (7). Both high levels of TS mRNA and TS protein in malignant tissue from metastatic disease sites predict poor response to 5-FU–based therapy (8). Insufficient TS inhibition may represent a major mechanism of resistance to FU or other TS inhibitors. In addition to intrinsic high basal TS content, which could impede sufficient TS inhibition, increased levels of TS (TS upregulation) in cells exposed to TS inhibitors through TS autoregulatory translational feedback loop present another mechanism of acquired resistance to TS inhibitors (9,10). The use of antisense oligodeoxynucleotides (ODN) targeting TS could downregulate TS mRNA and protein levels and increase the effective drug-to-target ratio for a more efficient TS inhibition (11,12).
Using the strategy of antisense TS to suppress TS, a target enzyme of 5-FU, and the enhancement of DNA IdUrd incorporation by stronger TS inhibition, we have designed an in vivo electrogene transfer (EGT) model using antisense TS plasmid (ATS) as a molecular modulation agent to increase tumor uptake of radiolabeled IdUrd. The combination of EGT ATS, 5-FU, and radiolabeled IdUrd is synergistic.
MATERIALS AND METHODS
Chemicals
The 5-FU (Sigma), 15 mg/mL, was freshly prepared in phosphate-buffered saline (PBS) before experiments. 125I-IdUrd was synthesized from IdUrd (Sigma) in our laboratory using previously published methods (13). Briefly, 100 μL oxidizing agent (H2O2:1N HCl:H2O = 4:1:95) were added to a 300-μL v-vial coated with 50 μg (0.1 μmol/L) 5-tributylstannyl-2′-deoxyuridine and containing 20 μL ethanol and 3.7 MBq sodium 131I-iodide. The reaction mixture was set aside and stirred intermittently with a vortex mixer. After 8 min, the mixture was frozen in liquid nitrogen and lyophilized under vacuum for about 1 h to produce the final product as a hot kit. Unreacted 131I-iodide, oxidizing agent (H2O2), and HCl were volatile and removed during freeze drying. The lyophilized 131I-IdUrd hot kit was redissolved in ethanol, and the radiochemical purity was determined using thin-layer chromatography and high-performance liquid chromatography. The labeling yield was >95%, and the radiochemical purity was >98%. The lyophilized 131I-IdUrd product was chemically stable up to 3 wk.
Cell Culture and Subcutaneous Implantation of CT26 Cells
CT26 is a murine colon adenocarcinoma cell line syngenic with BALB/c mice. CT26 cells (American Type Culture Collection) were routinely maintained in vitro in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL) with 10% fetal bovine serum (FBS) and antibiotics and incubated at 37°C in air containing 5% CO2. Female BALB/c mice, 4–6 wk old, were implanted with 1 × 105 cells in the right flank with a 27-gauge needle in a 1-mL tuberculin syringe. Approximately 10 d later, when tumors reached 4 × 4 mm in diameter, mice were randomly assigned to groups. Animals were maintained at the Animal Care Center of Veterans General Hospital, Taipei, Taiwan, and the experiment was approved by the Institutional Animal Care and Use Committee.
Plasmids
The ATS was constructed by inserting the cDNA sequence complementary to nucleotide 531-710 in the coding region of the mouse TS (mTS) mRNA into the plasmid vector pcDNA3 (Invitrogene). Numbering of bases was according to GenBank accession no. XM_131960. Polymerase chain reaction (PCR) was performed to synthesize the cDNA of mTS. After PCR amplification, mTS cDNA with HindIII/BamH1 restriction ends was inserted into a similarly restricted, cytomegalovirus (CMV)-promotor–based vector, pcDNA3. The plasmids used in this study were purified with a plasmid Mega kit (Qiagen).
In Vitro ATS Transfection
ATS expression vectors were transfected into CT26 cells using a BTX T820 square-wave pulse generator (Genetronics Inc.) in Ca2+- and Mg2+-free Hank’s balanced salt solution containing double-stranded expression vector at a final concentration of 1 μg/mL. Cells (2 × 105) were dispensed into 6-well tissue-culture plates and allowed to adhere overnight. Cells were washed once with 2 mL of DMEM, followed by electroporation. Cells were incubated for an additional 30 min at 37°C in a 5% CO2 incubator, washed once with serum-free DMEM, and left in 2 mL of DMEM with supplements. Control cells were transfected with pcDNA3/CMV vector without insert. Transiently transfected cells were used after 48 h.
Thymidylate Synthase Catalytic Activity Assay
The assay determined the catalytic activity of TS by means of tritiated water released during the TS-catalyzed conversion of 5-3H deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) (14). Cell suspension (4 × 106 cells/mL) in 50 mmol/L Tris-HCl buffer, pH 7.4, containing 2 mmol/L dithiothreitol was sonicated on ice (15 s at 23 kHz). Cell extracts were immediately centrifuged at 100,000g for 30 min at 4°C, and the protein concentration of supernatants was measured using a protein assay reagent (Bio-Rad). Cytosols were incubated with 3H-dUMP (100 nmol/L final concentration) and 5,10-methylene-5,6,7,8-tetrahydrofolate (0.63 mmol/L final concentration) in a total volume of 55 μL in Tris-HCl buffer. After 25 min of incubation at 37°C, the reaction was stopped on ice. Excess 3H-dUMP was removed by adding 300 μL of activated charcoal (15%) containing 4% trichloroacetic acid. After centrifugation at 5,000g for 10 min, the radioactivity present in 150-μL supernatants was determined by liquid scintillation counting (model LS 3801; Beckman Coulter).
Cell Cycle Analysis
CT26 cells transiently transfected with ATS or control plasmid for 48 h were trypsinized and washed twice with PBS. Cell pellets were suspended in 1 mL of 70% ethanol for 30 min at −20°C, 1 × 106 cells were centrifuged and resuspended in 1 mL of propidium iodide staining solution (0.04 mg/mL propidium iodide, 100 μg/mL DNase-free RNase A), and the mixture was incubated at 37°C for 20 min. Flow cytometric analysis was performed using a FACScan cytometer (Becton Dickinson).
In Vitro 131I-IdUrd DNA Incorporation
To assess the effect of ATS on the incorporation of 131I-IdUrd into nucleic acid, the cells transfected with 1 μg of the ATS were exposed for 3 h to 0.37 MBq/mL 131I-IdUrd 45 h after transfection. The cells were harvested, and the remaining isotopes were washed 3 times with PBS and pelleted. The cells were extracted twice with 100 μL of 0.2N perchloric acid. The insoluble material was incubated at 37°C for 20 min with 100 μL RNase solution. The reaction was terminated by the addition of 200 μL of 0.2N perchloric acid and centrifuged at 14,000 rpm for 10 min. The pellet was then counted in a scintillation counter (IS-330; Beckman).
In Vivo EGT
Groups of mice were assigned to 7 groups: control (EGT control plasmid + PBS pump); ATS control (EGT ATS + PBS pump); 131I-IdUrd control (EGT control plasmid + 131I-IdUrd pump); 5-FU control (EGT control plasmid + 5-FU + PBS pump); EGT ATS and 131I-IdUrd pump; EGT ATS and 5-FU (EGT ATS + 5-FU + PBS pump); and EGT ATS, 5-FU, and 131I-IdUrd pump. Plasmids were delivered by EGT. EGT treatments were performed on mice anesthetized by intraperitoneally injected ketamine. Plasmids were injected slowly into tumor using a 30-gauge needle with needle direction rotated for uniform distribution in the tumor. A lapse of 5 min was maintained between the gene injection and the application of electric pulses to allow plasmids to spread uniformly throughout the tumor. Electric pulses were generated by a BTX T820 square-wave pulse generator (Genetronics, Inc.) through a specially designed needle-array electrode, consisting of 6 needles arranged along the circumference of a circle 1 cm in diameter. Six 99-μs pulses of 1,300 V each, spaced at an interval of 10 s, were applied between 2 opposite pairs of the 6-needle array using manual switching. After each pulsing, the switch with the needle array was rotated through an angle of 60°, thus allowing uniform pulsing throughout the tumor volume after 6 pulses. EGT ATS was administered 3 times (on days 1, 2, and 3) after tumors grew to 4 mm in diameter. 131I-IdUrd was delivered using osmotic minipumps (1 μL/h for 7 d; Alzet) at a dose of 11.1 MBq in normal saline starting on day 3. The minipumps were implanted subcutaneously in the left flank of mice, with a connecting cannula into the center of the tumor. The 5-FU (50 mg/kg) was administered by intraperitoneal injection on day 3. Each group in each experiment included 5 mice, and the experiment was repeated twice in each group. Tumors were measured using calipers, and tumor volumes were calculated (tumor volume = length × width2/2) every 2–3 d.
Biodistribution of 131I-IdUrd in Tumor by γ-Counting
Mice were killed with sodium pentobarbital 14 d after implantation of the miniosmotic pump. Whole tumors were taken with adhering normal tissues carefully removed, cut into pieces (about 0.1 g/piece), and weighed. Two milliliters of enzyme cocktail solubilizer (Soluene-350; Packard BioScience) were added to each sample and incubated at 50°C for 3 h to achieve tissue lysis. After complete lysis of the tissue, equal volumes of lysed tissue were transferred for γ-counting. Total radioactivity expressed as count per minute (cpm) and percentage of injected dose per gram (%ID/g) of tumor were recorded. The EGT control plasmid + 131I-IdUrd pump; EGT ATS + 131I-IdUrd pump; and EGT ATS, 5-FU, and 131I-IdUrd pump groups were compared.
Statistical Analysis
All results were expressed as mean ± SD and were analyzed by a 2-tailed Student t test or by 1-way ANOVA. Differences were considered statistically significant at P < 0.05.
RESULTS
In Vitro EGT ATS Induces S-Phase Block in CT26 Cells
Flow cytometric analysis was used to examine the cell cycle distribution of CT26 cells 48 h after in vitro EGT ATS. There were substantial increases in the fraction of cells in S phase (63.3% after treatment with ATS vs. 28.9% before treatment) and concomitant reduction in cells in the G0–G1 phase (34.5% after treatment vs. 64.6% before) (Fig. 1). This indicated that TS depletion in CT26 cells resulted in S-phase arrest. On the other hand, ATS seemed not to hamper the treated cells across the G2–M–G1 and G1–S phases.
Cell cycle changes 48 h after EGT ATS on CT26 cells. Representative flow cytometry graphs of cells 48 h after in vitro EGT with control plasmid (A), and cells 48 h after in vitro EGT with ATS (B). There was an increased S-phase blockage of CT26 cells 48 h after EGT ATS.
Effect of In Vitro EGT ATS on TS Activity in CT26 cells
The catalytic activity of TS was examined by measurement of radioactivity from tritiated water released during the TS-catalyzed conversion of 5-3H-dUMP to -dTMP.
The TS catalytic activity of CT 26 cells was decreased 40% 48 h after ATS transfection compared with cells treated with the control plasmid (Fig. 2).
Thymidylate synthase assay 48 h after in vitro EGT ATS on CT26 cells. TS catalytic activity was determined as described and was measured in femtomoles per milligram per hour. The TS catalytic activity of CT26 cells was decreased 40% 48 h after in vitro EGT with ATS compared with cells treated with the control plasmid. Data represent mean data of 3 experiments. All data shown are mean ± SD.
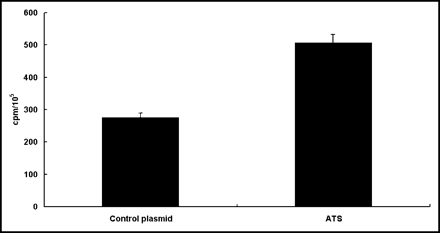
Effect of In Vitro EGT ATS on 131I-IdUrd DNA Incorporation in CT26 Cells
To assess the effect of ATS on the incorporation of 131I-IdUrd into DNA, CT 26 cells were transfected with ATS and then exposed to 3 h of 131I-IdUrd 45 h after electroporation. As shown in Figure 3, the radioactivity in the PCA-precipitated DNA was increased from 268 ± 20 cpm in the control plasmid transfected cells to 502 ± 31 cpm in the ATS transfected cells (P < 0.01).
Effect of in vitro EGT ATS on incorporation of 131I-IdUrd DNA in CT26 cells. CT26 cells were treated with either control plasmid or ATS by in vitro electroporation 45 h before adding 0.37 MBq/mL 131I-IdUrd for another 3 h. Counted to equal cell number, DNA was extracted by PCA precipitation, and pellets were counted for radioactivity. Bars represent mean ± SD of 3 independent experiments in triplicate. Results are represented as counts per minute per 105 cells.
Effect of In Vivo EGT ATS on CT26 Tumor Growth
Growth inhibition of CT26 tumor was examined to determine the antitumor efficacy of EGT ATS as a single-agent therapy or in combination. Treatment protocols were initiated 10 d after tumor inoculation, when subcutaneous tumor became palpable at around 4 × 4 mm. Groups of mice were assigned into 7 groups, including EGT control plasmid alone; EGT ATS alone; 131I-IdUrd alone; 5-FU alone; EGT ATS and 131I-IdUrd; EGT ATS and 5-FU; and EGT ATS, 5-FU, and 131I-IdUrd. Mice treated with EGT ATS alone or 131I-IdUrd alone showed a small growth delay when compared with control mice, but the difference was without statistical significance. The combination of EGT ATS and 131I-IdUrd produced a significant growth inhibition when compared with the 131I-IdUrd–alone group (P < 0.05) (Fig. 4). The combination of EGT ATS and 5-FU significantly retarded the tumor growth rate when compared with the 5-FU–alone group (P < 0.05) (Fig. 4). Three of 5 mice treated with a combination of EGT ATS, 5-FU, and 131I-IdUrd showed no tumor regrowth over 4 wk.
In vivo EGT ATS on the enhancement of antitumoral activity of 131I-IdUrd or 5-FU against established CT26 tumor. Mice bearing subcutaneous tumors 4 mm in diameter were randomized into 7 groups and treated in situ with EGT of control plasmid or ATS on days 1, 2, and 3, with or without 50 mg/kg 5-FU injected intraperitoneally on day 3, with implantation of osmotic minipumps containing 11.1 MBq 131I-IdUrd or 200 μL PBS from day 3 to day 10. Each symbol represents mean ± SD tumor volume from 5 mice per group. *P < 0.05.
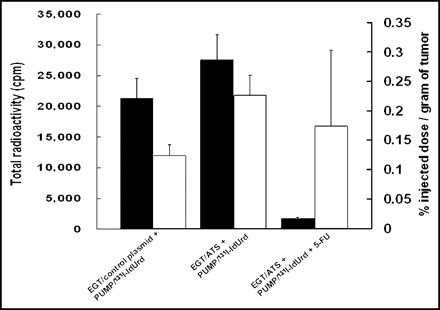
Effect of In Vivo EGT ATS on Retention of 131I-IdUrd Radioactivity in Tumor
Figure 5 shows the radiobiodistribution of 131I-IdUrd in CT26 tumor 14 d after treatment with infusion 131I-IdUrd with or without EGT ATS or 5-FU. An increase in the total radioactivity was found in the EGT ATS + 131I-IdUrd treatment group compared with the 131I-IdUrd–alone control group. The %ID/g of tumor was increased 91% in the EGT ATS treatment group (0.23% vs. 0.12% in the control group). The total radioactivity in the group treated with the combination of EGT ATS, 5-FU, and 131I-IdUrd, however, was markedly decreased as a result of the small tumor volume that remained 2 wk after the start of 131I-IdUrd injection. The %ID/g of tumor of radiolabeled IdUrd was lower in the EGT ATS, 5-FU, and 131I-IdUrd treatment group than in the EGT ATS and 131I-IdUrd treatment group. Because most of the tumors and surrounding normal tissues were indistinguishable macroscopically in the EGT ATS, 5-FU, and 131I-IdUrd treatment group, a large proportion of the weighted tumor sample in this group was, in fact, normal surrounding tissue that may provide an underestimation of the %ID/g.
Biodistribution of 131I-IdUrd in tumor 14 d after implantation of miniosmotic pumps. CT26 tumors were in vivo EGT with control plasmid or ATS for 3 d before implantation of 131I-IdUrd miniosmotic pump with or without 50 mg/kg 5-FU injected intraperitoneally on day 3. Black columns represent total radioactivity count for indicated treatment groups. White columns represent percentage injected dose per gram of tumor.
DISCUSSION
This study documented a molecular modulation strategy of downregulation of TS expression by EGT ATS. In vitro electroporation of ATS resulted in an effective suppression of the TS level in CT26 cells and led to S-phase arrest. In vivo ATS delivery by in situ EGT resulted in a slower growth rate of established tumors. The combination of in vivo EGT ATS and 131I-IdUrd resulted in higher radioactivity retained in tumor than did 131I-IdUrd alone. The combinations of in vivo EGT ATS, 5-FU, and 131I-IdUrd demonstrated a sustained effect of growth inhibition. Furthermore, 60% of mice in this group were alive and tumor free.
These results indicated that in vivo EGT ATS was effective. Transfer and expression of specific genes into tumor tissues is widely regarded as a valuable approach for investigating antitumor activities of specific genes. Most protocols use ex vivo modulation of target cells (15,16). Other approaches for in vivo gene transfer include viral vectors, cationic liposomes, and direct plasmid intramuscular injection (17–19). Although these techniques have been used successfully, they exhibit a variety of limitations, such as anatomic constraints, low transfection efficiencies, nonspecific targeting, inconsistent reproducibility, and safety concerns. Electroporation provides a promising solution for in vivo gene transfer. The combination of local injection of anticancer agent and in vivo electroporation, known as electrochemotherapy, was the pioneer in this field (20,21). In addition to small-molecular anticancer drugs, successful in vivo and in situ gene transfer into liver, skeletal muscle, and heart has also been reported (22–24).
There is a close association between increased TS expression and the development of TS inhibitor resistance (25,26). Both high levels of TS mRNA and TS protein in malignant tissue from metastatic disease sites predict poor response to 5-FU–based therapy (8). Insufficient TS inhibition may represent a major mechanism of resistance to 5-FU or other TS inhibitors (27,28). TS has been shown to bind its own mRNA and inhibit its translation. Treatment with 5-FU can induce a rebound of TS expression through the inhibition of a TS autoregulatory translational feedback loop (9,10,29). Improved cytotoxic efficacy of 5-FU may be achieved by ATS treatment through downregulation of intrinsic basal TS expression and the rebounded TS expression from 5-FU treatment.
Ferguson et al. (30,31) demonstrated that antisense ODN (20mers) directed against TS mRNA could enhance the efficacy of 5-FdUrd in parent and drug-resistant HeLa cells. Ju et al. (32) reported that antisense ODN (18mers) against TS decreased TS catalytic activity in cells in a dose-dependent manner over a short period but that the long-term effect of the TS antisense ODN treatment induced resistance to 5-FdUrd, possibly as a result of a rebound in TS expression after an initial transient TS mRNA suppression. When these researchers transfected plasmid containing long antisense fragments (+1–+422) of TS, long-term suppression of TS and markedly enhanced FdUrd sensitivity were noted. The superiority of an antisense strategy using plasmids with longer antisense sequences than conventional ODN may be explained by the fact that antisense ODN can bind only briefly with TS mRNA. A rapid degradation of the ODN in cells will result in a rebound of TS activity. The endogenous expression of ATS from plasmid can continuously inhibit the TS mRNA because of the longer half-life of plasmid in cells. Similarly, Schmitz et al. (33) have demonstrated that a 2′-O-methyl RNA oligoribonucleotide (ORN) antisense strategy not only targeted the TS mRNA but also prevented the acute induction of TS during TS inhibitor treatment. This may also be because this type of ORN is more resistant to nuclease degradation by RNase. These researchers found that the introduction of antisense TS into the cell results in an increase of p53 protein (33). TS protein has been reported to bind to the mRNA of at least 9 other proteins important in cell cycling and resistance to toxicity, including p53, c-myc, and others (34). The induction of p53 protein could permit easier induction of cell death by cytotoxic agents (35). Therefore, ATS gene therapy could be advantageous in many ways. First, it could be used as an alternative drug for TS inhibition. Second, it could serve as a sensitizer of TS inhibition by decreasing the target enzyme and preventing acquired drug resistance by suppressing the synthesis of new TS. Third, the p53 protein induction from downregulation of TS may aid in apoptosis induction.
Although ATS gene therapy might increase 5-FU sensitivity, it would be difficult to cure tumors with high TS expression using a combination of ATS and 5-FU therapy alone. Radiolabeled IdUrd is an ideal drug, taking advantage of TS inhibition in combination with 5-FU. An Auger electron-emitter, such as 131I, is toxic to cells after incorporation into DNA. The β-emitter 131I has a wider range of killing, which may provide crossfire irradiation between cells (3,4). Therapeutic use of combined-energy 131I- and 125I-IdUrd may efficiently kill cancer cells in cycling or noncycling if a relatively high dose can be achieved under strong and sustained TS inhibition.
A potential consequence of the administration of 131I-IdUrd is radiation damage to normal proliferating tissues, such as bone marrow and gut. Fortunately, IdUrd deiodinates rapidly in vivo, with a half-life of <5 min (36). It would be particularly suitable in tumors in which locoregional delivery, such as intratumoral injection, intraarterial delivery, or intracavitary installation, is possible so that dehalogenation is prevented before presentation to the dividing cells. To date, a number of studies have confirmed minimal adverse effects of radiolabeled IdUrd on normal tissue, even when administered repeatedly and in relatively high doses (27,28,37–39). The present study was designed to increase the extent of cellular uptake of 131I-IdUrd in experimental tumors by using a miniosmotic pump. Mairs et al. (40) had reported that the osmotic pump was better than biodegradable implants in yielding a greater accumulation of radioactivity.
The success of EGT ATS and radiolabeled IdUrd therapy depends mainly on 2 requirements: applying the appropriate field pulses to the tumor sites at which target genes are distributed evenly by needle injection and achieving satisfactory tumor targeting of radiolabeled IdUrd. The latter is the focus of interest of many investigators, and the combination of ATS gene, 5-FU, and radiolabeled IdUrd is the solution that we have proposed. The clinical use of EGT is only in the beginning stages. Genes such as ATS and drugs such as 131I-IdUrd may be applied through the same applicators for electrical pulse delivery. The development of optimal gene application methods, electrical parameters, and appropriate applicators for EGT are needed before wider clinical efforts can be made.
CONCLUSION
Taken together, our results demonstrated that in vivo EGT ATS could be used as a molecular modulation strategy to increase tumor uptake of radiolabeled IdUrd. The combination of EGT ATS, 5-FU, and 131I-IdUrd treatment may result in tumor eradication.
Acknowledgments
The authors thank Dr. Shang-Hue Yen for his administrative support and Bor-Song Liaw and Fan-Wei Tseng for their excellent technical assistance. The study was supported by grant VGH 91-240 from Veterans General Hospital, Taiwan, and partially supported by grant NSC92-2745-P-075-001 from the National Science Council.
Footnotes
Received Jul. 28, 2003; revision accepted Nov. 3, 2003.
For correspondence or reprints contact: Kwan-Hwa Chi, MD, Department of Radiation Therapy and Oncology, Shin-Kong Wu Ho-Su Memorial Hospital, 95 Wen Chang Rd., Taipei 111, Taiwan.
E-mail: M006565{at}ms.skh.org.tw