Abstract
Bone marrow is the dose-limiting organ in targeted radionuclide therapy. Hence, determination of the absorbed dose to bone marrow from incorporated radionuclides is a critical element in treatment planning. This study investigated the potential of the micronucleus assay in peripheral blood reticulocytes (MnRETs) as an in vivo biologic dosimeter for bone marrow. Methods: After intravenous administration of 32P-orthophosphate or 90Y-citrate in Swiss Webster mice, DNA damage induced in bone marrow erythroblastoid cells was measured by subsequent scoring of MnRETs in peripheral blood. The response to exponentially decreasing dose rates was calibrated by irradiating animals with external 137Cs–γ-rays. The γ-ray dose rate was decreased exponentially, with the dose-rate decrease half-time corresponding to the effective clearance half-time (Te) of the radioactivity from the femoral bone (Te = 64 h for 90Y-citrate and Te = 255 h for 32P-orthophosphate). Results: The maximum MnRETs frequency occurred on the second and third day after injection of 90Y-citrate and 32P-orthophosphate, respectively. The same pattern was observed for exponentially decreasing dose rates of 137Cs–γ-rays. For each type of exposure, the maximum MnRETs frequency increased in a dose-dependent manner. Using the calibrated dosimeter, the initial dose rates to the marrow per unit of injected activity were 0.0020 cGy/h/kBq and 0.0026 cGy/h/kBq for 32P-orthophosphate and 90Y-citrate, respectively. Conclusion: Micronuclei in peripheral blood reticulocytes can be used as a noninvasive biologic dosimeter for measuring absorbed dose rate and absorbed dose to bone marrow from incorporated radionuclides.
Bone marrow is generally recognized to be the dose-limiting organ in targeted therapies such as radioimmunotherapy or palliation of metastatic bone pain with radionuclides (1,2). Consequently, estimation of the absorbed dose to bone marrow is a critical element in planning targeted radionuclide therapy. Today, the most widely used methods for estimating the absorbed dose to bone marrow from radiopharmaceuticals used in radioimmunotherapy are based on the organ S value approach developed by the MIRD Committee (3). This approach relies on calculations based on standard models of the body (4), biokinetics data (5), and assumptions regarding the relative concentrations of radioactivity in the blood and bone marrow (6). However, despite intensive efforts, computational approaches to bone marrow dosimetry have not been greatly successful at correlating the marrow response with absorbed dose. The reasons for this are many and include wide variations in patient anatomy relative to the theoretic models used, variations in relative uptake of radioactivity in the blood and marrow, dose-rate effects, proliferation, bone marrow reserve, and the different radiation properties of the radionuclides used (7–11). Some of these complexities have been incorporated into various dosimetry approaches, leading to modest improvement in correlation with marrow response (12,13). These efforts have brought about a call for dosimetry techniques that are even more patient specific.
One can argue that the most patient-specific method for dose estimation would be a biologic dosimeter. The complexities of individually calibrating a biologic dosimeter for each patient make the method impractical at present; however, biologic dosimetry can be used to verify and validate computational approaches. In addition, biologic dosimetry for bone marrow can be useful in animal experiments. Recently, survival of granulocyte–macrophage colony-forming cells (GM-CFC) was used as a biologic dosimeter to experimentally determine the dose-rate kinetics in murine bone marrow from incorporated 90Y, 33P, and 32P (9,14). Although GM-CFC survival is an accurate biologic dosimeter for doses higher than 1 Gy, at which cell killing predominates, the method is invasive. This problem also exists with survival assays based on more primitive marrow cells (15,16). Therefore, the use of GM-CFC as a biologic dosimeter is limited for practical reasons.
Radiation-induced changes have been detected in peripheral blood cells at molecular, cytogenetic, and cellular levels. Chromosomal aberrations and micronuclei are commonly used cytogenetic endpoints. Evaluation of chromosomal aberrations in peripheral blood lymphocytes has provided valuable information on radiation exposure. This technique is the most frequently used method for biologic dosimetry (17–20). On the basis of strong evidence that the chromosomal aberration yield is proportional to absorbed dose, the International Atomic Energy Agency has proposed chromosomal aberration yield as a biologic dosimeter for retrospective dose assessment in radiation accidents (21).
The micronucleus assay has also effectively been used as an alternative to chromosomal aberrations for evaluation of the genotoxic or clastogenic effects of chemical and radiation exposure. Micronuclei arise from acentric fragments, or whole chromosomes, that fail to incorporate into the daughter nuclei during mitosis. Consequently, enumeration of micronuclei in the cytoplasm of the interphase daughter cell has been used to quantitate clastogenic or aneugenic chromosome DNA damage. Early efforts to detect micronuclei in bone marrow cells were reported (22,23). Micronuclei have been found in the erythrocytes of mouse peripheral blood, and the use of this noninvasive peripheral blood micronucleus assay as an alternative to invasive bone marrow assays has been shown possible (24,25). Recently, induction of micronuclei in peripheral blood reticulocytes (MnRETs) has been used as a biologic dosimeter in mice exposed to x-rays (26). Validation of the MnRETs test for assessing the mutagenic potential of well-known chemical mutagens has also been reported (27).
In this article, a noninvasive in vivo method is presented, namely induction of MnRETs in peripheral blood of mice, as a potential biologic dosimeter for determining the absorbed dose to the bone marrow from incorporated radionuclides. Studies were performed with 32P-orthophosphate or 90Y-citrate. Both radionuclides are high-energy β emitters with maximum energies of 1.71 and 2.28 MeV, respectively. These radionuclides were selected because 90Y is commonly in use and because 32P was recently proposed as a promising isotope for radioimmunotherapy (7,8).
MATERIALS AND METHODS
Animals
Female Swiss Webster mice 5–6 wk old were purchased from Taconic Farms (Germantown, NY) and used for our experiments after 1 wk of acclimation to the new housing conditions. During the study, commercial mouse chow and purified water were provided ad libitum. Each experimental and control group consisted of a minimum of three animals.
Radionuclide Administration and Biokinetics
The radionuclides were obtained in no-carrier added form (New England Nuclear, Boston, MA). 32P as orthophosphoric acid in water was diluted in phosphate-buffered saline and injected intravenously as a bolus containing activities of 185, 629, and 1221 kBq (experiment I) and 222, 655, and 1,110 kBq (experiment II). The radionuclide 90Y was obtained as yttrium chloride in 0.05 N HCl, was buffered with 0.1 mol/L sodium citrate (pH 7.0), was diluted with phosphate-buffered saline, and was injected intravenously as a bolus containing activities of 370, 1,110, and 1,665 kBq (experiment I) and 370 and 1,110 kBq (experiment II). All intravenous administrations were a 200-μL bolus through the tail vein.
Separate experiments were performed to ascertain the biokinetics of 32P-orthophosphate and 90Y-citrate in the femurs and various major organs (9,14). The effective clearance half-time (Te) values from the femoral bone were 255 and 64 h, respectively. Dosimetry calculations indicated that more than 90% of the absorbed dose received by the femoral marrow was from bone activity (9,14).
Experimental Model
The MnRETs in peripheral blood of mice were used as an in vivo biologic dosimeter for bone marrow. The procedures of the assay were described in detail earlier (26,28). Briefly, blood samples were collected immediately before treatment (0 d = control) and 1, 2, 3, 4, 6, 7, 8, 10, 14, 21, 28, and 35 d after 32P administration or 0, 1, 2, 3, 4, 6, 8, 11, and 14 d after 90Y injection. Blood (5 μL) obtained without anticoagulant from a tail vein was placed on a microscope slide coated with acridine orange (C.I. 46005, 10 μL at 1 mg/mL; Fisher Scientific Co., Fairlawn, NJ). A cover slip was immediately placed on the drop of blood. The cells were allowed to settle overnight at 4°C in a humidified chamber. The cytogenetic analysis was performed with a BH2 epifluorescence microscope (Olympus, Tokyo, Japan). Only reticulocytes of types I, II, and III, as classified by Vander et al. (29) according to RNA content, were scored. One thousand randomly selected reticulocytes from each mouse were scored for the number of micronuclei (greenish yellow) in each. The cells with only fluorescing dots were excluded from scoring. All scoring was by a single observer using coded slides.
Calibration of the Biologic Dosimeter
The biologic dosimeter was calibrated using our custom-designed low-dose-rate 137Cs irradiator (equipped with a computer-controlled mercury attenuator system), which facilitated the delivery of exponentially decreasing dose rates of γ-rays (30). This irradiator allowed simultaneous irradiation of mice with different initial dose rates (ro) by placement of different groups of mice at different distances from the 137Cs source. Although the ro values were different for each group of mice, the dose rates were exponentially decreased using a predetermined dose-rate decrease half-time, Td, the time required for the dose rate to decrease by half (30). In groups of three or more, the mice were caged on different shelves in the irradiator cabinet, and the computer-driven mercury attenuator was programmed to deliver predefined ro values to each cage and then decrease the dose rates with a fixed Td. The dose rates and total doses to each cage were monitored during irradiation using MOSFET dosimeter probes customized for low-dose-rate measurements (Thomson Nielsen Electronics Ltd., Ottawa, Ontario, Canada). On various days during the irradiation process, the animals were briefly removed from the irradiator and a blood sample was obtained for assay of MnRETs. Two Td values (64 and 255 h) were used for these studies. These Td values corresponded to the Te values of 90Y-citrate (14) and 32P-orthophosphate in the femurs (9). The ro values from the bottom cage to the top cage were 0.635, 1.33, and 2.86 cGy/h for a Td of 64 h and 0.10, 0.17, 0.36, and 1.05 cGy/h for a Td of 255 h.
RESULTS
Biologic Response After Radionuclide Administration
The average frequency of MnRETs induced as a function of injected activity (AInj) and time after injection is shown in Figures 1 and 2 for 32P and 90Y, respectively. 32P-orthophosphate induced MnRETs frequencies of 0.76%, 2.12%, and 3.03% after injection of 203, 642, and 1,165 kBq, respectively. Similarly, 90Y yielded MnRETs frequencies of 1.33% and 2.28% after injection of 370 and 1,110 kBq, respectively. An administered activity of 1,665 kBq 90Y resulted in severe depletion of erythrocytes, with no reticulocytes observed on days 1 and 2 after injection (Fig. 2). No depletion effects were observed for the 32P injected activities.
Frequency of induction of MnRETs in peripheral blood of mice as function of time after intravenous injection of 32P-orthophosphate. Results are shown for three different injected activities. Error bars represent SEs of mean for two independent experiments.
Frequency of induction of MnRETs in peripheral blood of mice as function of time after intravenous injection of 90Y-citrate. Response curves are shown for three different injected activities. Error bars represent SEs of mean for two independent experiments.
The spontaneous incidence of MnRETs established for blood samples collected immediately before treatment (0 d = control) ranged from 0.17% to 0.32% for all experiments performed, with a mean value of 0.22% ± 0.08%.
Biologic Response After Chronic γ-Irradiation
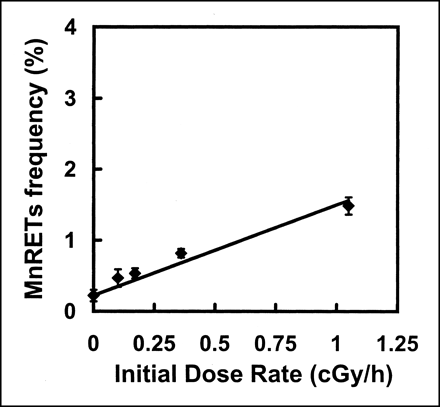
The results on MnRETs frequency as a function of initial dose rate (cGy/h) and time after initiating irradiation by chronic external 137Cs–γ-rays with exponentially decreasing dose rates are presented in Figures 3 and 4. The Td values of 255 and 64 h were set to simulate the bone marrow dose-rate kinetics for 32P and 90Y injection, respectively. The days on which the maximum induced MnRETs were observed were the same as for the animals injected with radionuclides. That is, for chronic exposure to γ-rays with a Td of 255 h (simulated 32P kinetics in the femur), the maximum occurred on the third day after initiating the irradiation. On that day, mean MnRETs frequencies of 0.37%, 0.43%, 0.92%, and 2.07% were induced by ro values of 0.10, 0.17, 0.36, and 1.05 cGy/h, respectively. When the dose rate was decreased, with Td = 62 h (simulated 90Y kinetics in the femur), the maximum was on the second day. On that day, mean MnRETs frequencies of 0.82%, 1.50%, and 2.23% were obtained for ro values of 0.635, 1.33, and 2.86 cGy/h, respectively.
Frequency of induction of MnRETs in peripheral blood of mice as function of time after initiating irradiation with external γ-rays from 137Cs irradiator. Dose rate was decreased exponentially, with Td of 255 h, which corresponds to Te from femurs of mice that were injected with 32P-orthophosphate. Data points represent mean ± SE of mean of two independent experiments.
Frequency of induction of MnRETs in peripheral blood of mice as function of time after initiating irradiation with external γ-rays from 137Cs irradiator. Dose rate was decreased exponentially, with Td of 64 h, which corresponds to Te of radioactivity from the femurs of mice that were injected with 90Y-citrate. Data points represent mean ± SE of mean of two independent experiments.
MnRETs Frequency as a Function of AInj
The MnRETs frequency on the day of maximum response is shown as a function of AInj in Figures 5 and 6 for 32P and 90Y, respectively. Least squares fits of the data to a linear function yielded the following relationships:
 1
1
 2
where %MnRETs is the percentage of reticulocytes with micronuclei and AInj is in kilobecquerels. The intercept of 0.22% represents the mean background value on day 0 for all experiments performed. The R2 values for these fits were 0.98 and 0.92, respectively.
2
where %MnRETs is the percentage of reticulocytes with micronuclei and AInj is in kilobecquerels. The intercept of 0.22% represents the mean background value on day 0 for all experiments performed. The R2 values for these fits were 0.98 and 0.92, respectively.
Frequency of induction of MnRETs in peripheral blood of mice as function of AInj of 32P-orthophosphate on third day after administration. Error bars represent SEs of mean for two independent experiments.
Frequency of induction of MnRETs in peripheral blood of mice as function of AInj of 90Y-citrate on second day after administration. Error bars represent SEs of mean for two independent experiments.
Calibration of the Biologic Dosimeter
Figures 7 and 8 show the MnRETs induced as a function of initial dose rate (cGy/h) of 137Cs–γ-rays. Least squares fits of the data to a linear function yielded the following:
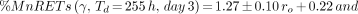 3
3
 4where ro is in cGy/h. The R2 values for these fits were 0.95 and 0.95, respectively.
4where ro is in cGy/h. The R2 values for these fits were 0.95 and 0.95, respectively.
Frequency of induction of MnRETs in peripheral blood of mice as function of initial dose rate from 137Cs–γ-rays on third day after initiating irradiation. External γ-rays were delivered with exponentially decreasing dose rate, with Td of 255 h. Error bars represent SEs of mean for two independent experiments.
Frequency of induction of MnRETs in peripheral blood of mice as function of initial dose rate from 137Cs–γ-rays on second day after initiating irradiation. External γ-rays were delivered with exponentially decreasing dose rate, with Td of 64 h. Error bars represent SEs of mean for two independent experiments.
Initial Dose Rate and Dose to Marrow as a Function of AInj
The initial dose rate to the marrow per unit of AInj can be estimated for 32P-orthophosphate from the ratio of the slopes presented in Equations 1 and 3 and for 90Y-citrate from Equations 2 and 4. The values were, therefore,
 5
5
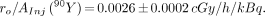 6
6
The absorbed dose delivered to the marrow at the time when the maximum MnRETs frequency was observed was obtained by integrating the dose rate (r(t) = ro exp(−0.693 t/Te)). Integrating over 3 and 2 d for 32P-orthophosphate and 90Y-citrate, respectively, gave:
 7
7
 8
8
Had these integrations been performed to infinity, the values would be 0.734 and 0.239 cGy/kBq, respectively. The absorbed dose required to produce a micronucleus frequency equal to that which occurs spontaneously is called the doubling dose. Using Equations 1 and 2, the injected activities required to double the MnRETs frequency were 88 and 110 kBq for 32P and 90Y, respectively. Equations 7 and 8 give doubling doses of 11.5 and 10.6 cGy, respectively.
DISCUSSION
Our study explores the potential of the MnRETs assay to serve as a biologic dosimeter for bone marrow so that the impact of exposing this critical organ to incorporated radionuclides can be evaluated. Figures 1 and 2 show the induction frequency of MnRETs as a function of time after administration of 32P-orthophosphate and 90Y-citrate, respectively. In each case, the induction frequency increased sharply, with a maximum observed on the third and second days, respectively, after injection. Subsequently, the MnRETs frequency dropped as the radioactivity in the bone cleared, with a corresponding drop in the dose rate. The same pattern emerges when the animals are irradiated with exponentially decreasing dose rates of 137Cs–γ-rays (Figs. 3 and 4). If one defines TMn to be the time required for the MnRETs frequency to drop to half its maximum value, then the data on the descending portion of the graphs in Figures 1–4 can be fitted to an exponential function of the form:
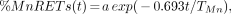 9
where a is the extrapolated %MnRETs (not used in the current analysis) and t is the time elapsed after the maximum was attained. The results of these fits are given in Table 1 for representative data from Figures 1–4. For 32P-orthophosphate, the radioactivity clears from the mouse femurs with a Te of 255 h (9), and the average TMn is 293 h. In the case of 90Y-citrate, for which the Te is 64 h, the average TMn = 89 h. When 137Cs–γ-rays are delivered with a Td of 64 h, approximately the same average value is obtained for TMn (88 h), whereas an average TMn of 253 h is obtained when Td is 255 h. These data indicate that the drop in %MnRETs essentially parallels the drop in dose rate to the marrow.
9
where a is the extrapolated %MnRETs (not used in the current analysis) and t is the time elapsed after the maximum was attained. The results of these fits are given in Table 1 for representative data from Figures 1–4. For 32P-orthophosphate, the radioactivity clears from the mouse femurs with a Te of 255 h (9), and the average TMn is 293 h. In the case of 90Y-citrate, for which the Te is 64 h, the average TMn = 89 h. When 137Cs–γ-rays are delivered with a Td of 64 h, approximately the same average value is obtained for TMn (88 h), whereas an average TMn of 253 h is obtained when Td is 255 h. These data indicate that the drop in %MnRETs essentially parallels the drop in dose rate to the marrow.
Comparison of Half-Times
When properly calibrated, %MnRETs can be used as a biologic dosimeter for incorporated radionuclides. This calibration process has been used to arrive at Equations 5 and 6, which give the initial dose rate to the bone marrow per unit AInj. The values obtained for 32P-orthophosphate and 90Y-citrate are 0.0020 ± 0.0004 and 0.0026 ± 0.0002 cGy/h/kBq, respectively. These values may be compared with those obtained for the same radiochemicals when survival of GM-CFC is used as the biologic dosimeter. For the GM-CFC survival endpoint, the corresponding values for ro/AInj are 0.0031 ± 0.0004 and 0.0030 ± 0.0003 cGy/h/kBq, respectively (14). Thus, the GM-CFC endpoint suggests that somewhat higher absorbed dose rates are delivered to the marrow per unit of AInj. However, several points must be kept in mind. The GM-CFC dosimeter actually provides the initial absorbed dose rate to the femoral marrow, whereas the MnRETs dosimeter gives the average initial absorbed dose rate to all the marrow in the body. This is because the GM-CFC data are derived from femoral marrow specifically, whereas the MnRETs emerge from marrow throughout the body and populate the peripheral blood. Given that both assays take into account all sources of marrow irradiation (self- and cross-dose), and that all marrow in the mouse is active marrow, one may ask why the values are different. One possible explanation is differential uptake of radioactivity in the various bones of the skeleton. The skeleton of the young mice used in these studies is in a state of rapid growth, particularly the femurs and other long bones (31). Therefore, these bone seekers may have been more actively concentrated in the femurs and tibias than in other bones. The result would be delivery of a higher absorbed dose to these regions compared with the skeleton as a whole. An alternate explanation may be that the two different radiosensitive cells in the marrow that are precursors to GM-CFC and reticulocytes do not receive the same absorbed dose rate. There is evidence that the numerous cell types in the marrow are not homogeneously distributed (32). Therefore, the precursors to the GM-CFC cells may receive a higher dose than the reticulocyte precursors. Alternatively, differences in the structure of the bone trabeculae in the femur versus other parts of the skeleton may also affect the absorbed dose (33,34). Experiments that use marrow from different skeletal compartments or use different marrow cells may help to resolve these questions.
The use of radiolabeled agents to deliver therapeutic absorbed doses to tumors is an attractive concept in targeted therapies such as radioimmunotherapy. Unfortunately, systemic administration of radiopharmaceuticals also irradiates the radiosensitive bone marrow cells. As a consequence, marrow is often the dose-limiting organ in such therapies. Therefore, knowledge of the absorbed dose rate and absorbed dose to the bone marrow is important in predicting the biologic response of this radiosensitive tissue. The considerable uncertainties in theoretic bone marrow dosimetry make biologic dosimeters an attractive alternative. The MnRETs assay offers several distinct advantages. The maximum %MnRETs occurs in a relatively short time after injection of the radiopharmaceutical, and the result can be obtained shortly after the blood is drawn. In contrast, colony-forming assays such as GM-CFC and cobblestone require longer times to reach their nadir, and the assays take 1–4 wk to complete. Furthermore, these colony-forming assays require many bone marrow cells. In the case of small animals such as mice, obtaining sufficient cells requires resection of one or more of the femurs or tibias, thereby necessitating killing of the animal. This is not desirable when one is following the status of tumor-bearing animals over extended times. Therefore, when properly calibrated, the MnRETs assay can be used to experimentally determine the absorbed dose to the marrow while continuing to follow tumor burden in the animals. This assay also has disadvantages as a biologic dosimeter. For example, when the marrow absorbed dose rates and doses are sufficiently high to cause a high degree of cell killing, finding enough reticulocytes to score can be difficult, as occurred in our study when 1,665 kBq 90Y-citrate was administered (Fig. 2). In that case, no reticulocytes were observed on days 1 and 2 after injection. Very few reticulocytes were observed on day 3 after injection, possibly interfering with determination of the MnRETs frequency. Therefore, caution must be exercised when doses are sufficiently high to kill a large fraction of the reticulocytes. Figure 1 suggests that reliable results are obtained with injected activities of at least 1,166 kBq 32P-orthophosphate. Using Equation 7, this activity corresponds to a marrow dose of approximately 1.5 Gy delivered during 3 d. Integration to infinity would result in a total marrow dose of 8.6 Gy. Figure 2 suggests that approximately 1,110 kBq 90Y-citrate is the limit for reliable results with this radiochemical. This AInj delivers a marrow dose of 1.1 Gy during 2 d. Integration to infinity yields 2.7 Gy. Therefore, higher absorbed doses can be accommodated for 32P-orthophosphate than for 90Y-citrate, likely because of the very different ro values that are required for these radiopharmaceuticals to deliver the same dose.
Our data indicate that induction of MnRETs can be used as a nondestructive biologic dosimeter for bone marrow in rodents. The question then arises of its potential for human use. Although reticulocytes have not been used as dosimeters in humans, a variety of reticulocyte assays have been established. For example, the maturity of reticulocytes can be assessed on the basis of the fluorescence staining intensity of reticulocytes, which is proportional to their RNA content. Automated discrimination of peripheral blood reticulocytes into youngest high-fluorescence reticulocytes, moderate-fluorescence reticulocytes, and oldest low-fluorescence reticulocytes is used in the clinical setting (35). The reticulocyte maturity index, represented by the mean fluorescence intensity (from all stages) of reticulocytes, is a valuable parameter that has proven effective in the early detection of bone marrow recovery after chemotherapy (36). The index is effective because high-fluorescence reticulocytes are an indicator of bone marrow recovery. Recently, Tsuji et al. (37) developed a rapid flow cytometric method (FCM) based on two-color staining with anti-CD45 fluorescein isothiocyanate and propidium iodide that is suitable for scoring nucleated red blood cells in peripheral blood specimens. FCM analysis of reticulocytes stained with acridine orange, thiazole orange, or proflavine can also be performed (38). Direct measurement of reticulocyte volume, hemoglobin concentration, and hemoglobin content are frequently used for real-time assessment of the functional state of the erythroid marrow. These examples show that analysis of reticulocytes can provide valuable clinical information. However, despite success as a mutagenic and genotoxic endpoint in rodents (39), interest in MnRETs for human use has not been substantial. Recently, a new FCM method to resolve micronucleated reticulocytes was reported by Torous et al. (40). Based on their patented method, evaluation of clastogenic and mutagenic effects induced by compounds using the peripheral blood of mice and rats is possible with a commercially available micronucleus kit. Given that the automated scoring procedure of Torous et al. has proven a rapid and efficient indicator of genotoxic and cytotoxic damage in rodents, a similar automated FCM for reticulocytes (or MnRETs) in humans, with potential applications for biologic dosimetry, may be possible.
CONCLUSION
Micronuclei in peripheral blood reticulocytes can be used as a biologic dosimeter for noninvasively measuring absorbed dose rate and absorbed dose to bone marrow from incorporated radionuclides. In targeted radionuclide therapy in rodents, this assay can be used to ascertain marrow absorbed dose without killing the animal, thus allowing following of the tumor burden.
Acknowledgments
The authors thank Harvey Ozer, Department of Microbiology and Molecular Genetics, for use of the fluorescence microscope. This study was supported in part by fellowship POL/93026 from the International Atomic Energy Agency, Vienna, Austria, and grant CA-54891 from the U.S. Public Health Service.
Footnotes
Received Apr. 5, 2000; revision accepted Jul. 31, 2000.
For correspondence or reprints contact: Roger W. Howell, PhD, Department of Radiology, MSB F-451, University of Medicine and Dentistry of New Jersey–New Jersey Medical School, 185 S. Orange Ave., Newark, NJ 07103.