Abstract
407
Background: Checkpoint inhibitor immunotherapy has greatly improved outcomes in patients with a variety of local and metastatic cancers, yet predicting early response to therapy has remained a challenge. In order to enhance efficacy, there is a profound need to better understand biological mechanisms of response and how immunotherapies alter the tumor microenvironment, including factors such as hypoxia and tumor vasculature. It has been demonstrated that molecular imaging can provide a noninvasive approach to accurately quantify hypoxia in vivo, and that these imaging metrics can be predictive of response to targeted therapies. Furthermore, hypoxia has been shown to have a negative effect on numerous anti-tumor immune cells, providing a rationale for quantifying this important component of the tumor microenvironment. In this study, we hypothesize that PET imaging of tumor hypoxia during treatment with immunotherapy could provide insight for predicting response.
Methods: MC38 murine colorectal cancer cells were subcutaneously injected into the upper right flank of C57BL/6 mice. Mice were treated with either 200 ug anti-mouse PD-1, a combination of 200 ug anti-mouse PD-1 and 200 ug anti-mouse CTLA-4 (n=6), or saline (n=7) via intraperitoneal injection every 3 days, for a total of 3 treatments, beginning 10 days following implantation. Tumor hypoxia was noninvasively imaged by injecting 5.6 MBq of [18F]-FMISO and performing PET imaging 80 minutes post-injection. PET imaging occurred prior to beginning therapy as well as 2 and 5 days post-therapy initiation. Tumor volume changes were longitudinally monitored for 2 weeks to quantify changes in tumor volume in response to immunotherapy. Mice were designated as non-responders and responders based on interim tumor volume analysis and the ratio of tumor to muscle [18F]-FMISO mean SUV was compared between groups. Statistical analysis was evaluated through a non-parametric T-test.
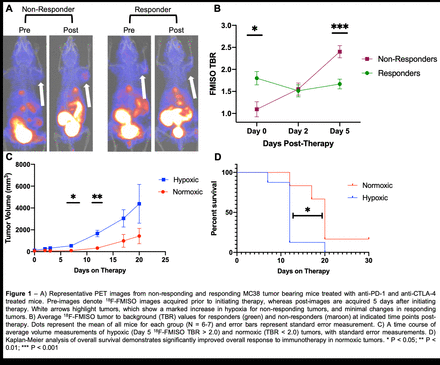
Results: Prior to initiating therapy, tumors that would subsequently respond demonstrated significantly higher levels of hypoxia as measured by tumor to muscle SUV ratio (tumor:background ratio - TBR) of 1.80 ± 0.37 compared to non-responding tumors, which measured a TBR of 1.09 ± 0.49 (P = 0.01). This trend was reversed following therapy, as tumor hypoxia increased in non-responders to a TBR of 2.40 ± 0.37, whereas hypoxia remained unchanged in responders (TBR = 1.67 ± 0.27). At day 5, tumors in the responding group had significantly lower hypoxia (P = 0.002) than non-responders. Separation of tumors into normoxic (TBR < 2.0) and hypoxic (TBR > 2.0) on day 5 post-therapy was predictive of both reduced tumor growth and increased overall survival. Mice identified as normoxic by [18F]-FMISO PET imaging had significantly smaller tumor volumes 12 days following treatment compared to the hypoxic group (330 ± 271 mm3 versus 1660 ± 836 mm3, repectively; P = 0.006). Additionally the median survival of normoxic tumors was 20 days, whereas hypoxic tumors had a median survival of only 12 days (P = 0.01).
Conclusions: Hypoxia PET imaging with [18F]-FMISO represents a valuable tool for measuring changes in the tumor microenvironment during immunotherapy and preliminary data shows potential for predicting eventual response to checkpoint blockade. Paradoxically, in the MC38 model, low hypoxia pre-treatment was correlated with resistance to therapy, whereas effective checkpoint blockade prevented the rapid onset of hypoxia during tumor progression. This imaging supports the hypothesis that the immune system plays an active role in promoting tumor vascularization, providing rationale for further investigation into the role of hypoxia PET imaging in guiding therapeutic decisions.