Abstract
609
Objectives: The adoptive transfer of tumor-targeting T cells offers a promising approach to treating tumors; however, clinical success has been limited. Activation and proliferation are key events after contact is made between the T cells and the tumor antigen. OX40 and GITR are co-stimulatory molecules, whose signaling is important for differential immune responses mediated by CD4 or CD8 T cells. In our preliminary testing, surface expression of OX40 and GITR on tumor antigen-specific T cells increased markedly following co-culture with tumor cells. The aim of this study was to investigate whether immune-PET imaging of OX40 and GITR enables non-invasive in vivo monitoring of functionally active T cells and, thus, whether it may assist in predicting therapeutic response in real time at an early stage.
Methods: We conjugated antibodies targeting OX40 and GITR via their lysine amino acids to 89Zr-DFO using isothiocyanate-bearing derivative of DFO. We utilized our established Rag1-/- mouse model lacking mature B and T cells engrafted with B16 melanoma, and injected melanoma-specific CD4-Trp1 T cells via the tail vein. Melanoma mice not receiving CD4-Trp1 T cells served as control groups. The mice underwent serial PET imaging following intravenous administration of 89Zr-DFO-OX40, -GITR and -IgG (isotype). We quantified the tracer uptake on PET and performed autoradiography and immunohistochemistry on the harvested tumor tissues.
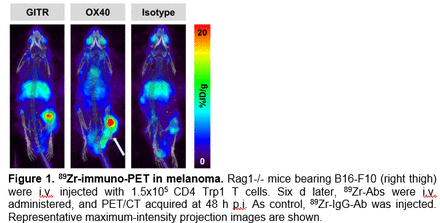
Results: 89Zr-DFO-conjugated antibodies specifically targeted their ligands OX40 and GITR in vivo as detected by immuno-PET and enabled non-invasive serial monitoring of the T cell surface activation markers at the tumor site. Tumor/T cell-to-normal organ ratios were favorable, resulting in high image contrast (Fig. 1, Table 1). Ex vivo autoradiography confirmed the specific binding of the radiolabeled antibodies. In contrast, uptake of 89Zr-DFO-OX40 and -GITR at the tumor site in control mice not injected with CD4-Trp1 T cells was minimal.
Conclusions: Preliminary results indicate that OX40 and GITR are up-regulated on CD4-Trp1 T cells at the tumor site, enabling specific binding of corresponding 89Zr-DFO-labelled antibodies in vivo. This approach is now being further developed to characterize the pharmacokinetics (PK) of marker expression over time and to investigate the association between OX40 and GITR expression at the tumor site and treatment efficacy. Monitoring the activation state of the T cells may also predict therapy success, individualize immunosuppressive therapy regimen, and, ultimately, optimize the design of new clinical trials.