Visual Abstract
Abstract
Chronic hypertension leads to injury and fibrosis in major organs. Fibroblast activation protein (FAP) is one of key molecules in tissue fibrosis, and 68Ga-labeled FAP inhibitor-46 (FAPI46) PET is a recently developed method for evaluating FAP. The aim of this study was to evaluate FAP expression and fibrosis in a hypertension model and to test the feasibility of 68Ga-FAPI46 PET in hypertension. Methods: Hypertension was induced in mice by angiotensin II infusion for 4 wk. 68Ga-FAPI46 biodistribution studies and PET scanning were conducted at 1, 2, and 4 wk after hypertension modeling, and uptake in the major organs was measured. The FAP expression and fibrosis formation of the heart and kidney tissues were analyzed and compared with 68Ga-FAPI46 uptake. Subgroups of the hypertension model underwent angiotensin receptor blocker administration and high-dose FAPI46 blocking, for comparison. As a preliminary human study, 68Ga-FAPI46 PET images of lung cancer patients were analyzed and compared between hypertension and control groups. Results: Uptake of 68Ga-FAPI46 in the heart and kidneys was significantly higher in the hypertension group than in the sham group as early as week 1 and decreased after week 2. The uptake was specifically blocked in the high-dose blocking study. Immunohistochemistry also revealed FAP expression in both heart and kidney tissues. However, overt fibrosis was observed in the heart, whereas it was absent from the kidneys. The angiotensin receptor blocker–treated group showed lower uptake in the heart and kidneys than did the hypertension group. In the pilot human study, renal uptake of 68Ga-FAPI46 significantly differed between the hypertension and control groups. Conclusion: In hypertension, FAP expression is increased in the heart and kidneys from the early phases and decreases over time. FAP expression appears to represent fibrosis activity preceding or underlying fibrotic tissue formation. 68Ga-FAPI46 PET has potential as an effective imaging method for evaluating FAP expression in progressive fibrosis by hypertension.
Hypertension is one of the main risk factors for major organ damage affecting the heart, kidneys, and brain (1–3). Chronic hypertension can lead to severe outcomes, including coronary artery disease, cerebrovascular disease, and renal failure, significantly increasing the risk of mortality from cardiovascular diseases (CVDs) and related conditions. A recent survey revealed that the prevalence of hypertension in the United States exceeds 100 million, with estimated annual health care costs of over $50 billion (4).
Organ fibrosis, one of the most common pathophysiologic results of hypertension, is caused by mechanical and biochemical stresses in hypertension. Progression of fibrosis leads to atherosclerosis in the vascular smooth muscles and cardiac remodeling in the myocardium, ultimately resulting in heart failure (5–8). Tissue fibrosis in the kidneys leads to renal dysfunction, including proteinuria and decreased glomerular filtration rate, and finally to chronic kidney disease (9–11). In this regard, noninvasive imaging of tissue fibrosis is promising in hypertension management. Although MRI is currently used in clinical practice for evaluating fibrosis, it is limited to established myocardial fibrosis (6).
Fibroblast activation protein (FAP) belongs to the type II dipeptidyl peptidase family of serine proteases, which is selectively expressed on the cell surface of activated fibroblasts. A FAP inhibitor (FAPI) is a substance that specifically binds to FAP, and FAPIs labeled with radioisotopes such as 68Ga and 18F have been developed as potential imaging tracers for PET. FAPI PET has proven valuable in diagnosing various types of cancer by targeting cancer-associated fibroblasts within tumors (12–15). Recently, there have been attempts to use FAPI PET in CVD, with increased FAP expression observed in myocardial infarction, heart failure, and chronic kidney disease (16–20).
In this study, we investigated the feasibility of 68Ga-FAPI-46 (FAPI46) PET to detect fibrosis of major organs in a mouse hypertension model induced by angiotensin II. The image findings of 68Ga-FAPI46 PET were compared with tissue characteristics during hypertension. Additionally, a pilot analysis of human 68Ga-FAPI46 PET was conducted in terms of hypertension.
MATERIALS AND METHODS
Animal Model
Details on the methods are presented in Supplemental Data 1 (supplemental materials are available at http://jnm.snmjournals.org). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Seoul National University (SNU-221124-2). Hypertension was induced in 8-wk-old male BALB/c mice by infusing angiotensin II (1.0 μg/kg/min; Sigma Aldrich) for 4 wk, via a subcutaneously implanted mini-osmotic pump. In the sham group, normal saline was infused using the same method. In a subgroup of hypertension, angiotensin receptor blocker (ARB) was treated using oral administration of losartan (10 mg/kg/d) for the same period as the angiotensin II infusion. Blood pressure, body weight, and serum levels of blood urea nitrogen and creatinine were measured to evaluate the adequacy of the animal model.
68Ga-FAPI46 Biodistribution Study
A 68Ga-FAPI46 biodistribution study was conducted at 1, 2, and 4 wk after hypertension modeling. 68Ga-FAPI46 was synthesized in house using a 68Ge/68Ga generator and DOTA-FAPI46. 68Ga-FAPI46 (0.37–1.85 MBq) was injected into the tail vein. Afterward, a renal clearance protocol that included hydration with normal saline and a furosemide injection was used to minimize radioactivity in the urine (21), as 68Ga-FAPI46 is excreted via the urinary system. The mice were euthanized at 60 min after 68Ga-FAPI46 injection, and major organs were harvested. The radioactivity of each organ tissue was measured by a γ-counter (2480 WIZARD2; PerkinElmer Inc.). The radioactivity was normalized by the tissue weight and expressed as the percentage injected dose per gram (%ID/g).
An additional biodistribution study was conducted at week 1, with high-dose (250 times the dose of 68Ga-FAPI46) DOTA-FAPI46 blocking to confirm FAP-specific uptake of 68Ga-FAPI46 in the tissues. Unlabeled high-dose DOTA-FAPI46 was coinjected into hypertensive mice, and the biodistribution study was performed in the same manner.
68Ga-FAPI46 PET/MRI Acquisition and Analysis
68Ga-FAPI46 PET/MRI was also performed at 1, 2, and 4 wk after hypertension modeling and additionally at week 1 with high-dose blocking. PET and MR images were simultaneously acquired using a hybrid system equipped with a small-animal PET scanner (SimPET; Brightonix Imaging Inc.) and a 1.0-T MRI scanner (M7; Aspect Imaging). 68Ga-FAPI46 (3.7–5.55 MBq) was injected into the tail vein, and the renal clearance protocol was also used. The mice were anesthetized with 2.0% isoflurane, and PET/MRI was performed 60 min after the injection. PET images were acquired for 10 min and reconstructed using an iterative algorithm (3-dimensional ordered-subset expectation maximization; 12 subsets and 3 iterations). MRI was performed with 3-dimensional gradient-echo sequences (voxel size, 0.25 × 0.25 × 1 mm; repetition time/echo time, 25/3.54 ms; excitation flip angle, 30°; slices, 25) for 15 min.
PET/MR images were analyzed using a commercial toolbox (MIM, version 7.1.8; MIM Software Inc.). To quantify 68Ga-FAPI46 uptake, 3-dimensional volumes of interest were drawn on the target organs including the heart, liver, and kidneys, based on the MRI. The uptake count of each organ was measured; to normalize the uptake value, SUV and target-to-background ratio (TBR) were calculated as the ratio between the maximum uptake count of the target organ and the mean uptake count of the liver.
Tissue Study for FAP Expression and Fibrosis
After the biodistribution study, the harvested heart and kidney tissues from the mice were fixed in 4% paraformaldehyde and embedded in paraffin. Masson trichrome staining was applied to these tissues to assess fibrosis. Immunohistochemistry staining was performed to detect target protein expression, using the primary antibody (anti-FAP [PA5-99313; Invitrogen], and antivimentin [ab92547; Abcam]).
Pilot Analysis of Human 68Ga-FAPI46 PET
68Ga-FAPI46 PET images obtained from a previous prospective study were analyzed as a pilot study. The original study was designed to assess the efficacy of 68Ga-FAPI46 PET in lung cancer. Twenty-eight patients were enrolled to undergo preoperative 68Ga-FAPI46 PET. From this cohort, patients with hypertension (≥grade 1) and a control group were chosen on the basis of the selection criteria. Renal uptake was quantified as SUVmean and TBR using the liver as the reference organ and was compared between the hypertension and control groups. Myocardial uptake was not discernible from blood-pool activity in the ventricle and was not measured. The methods for obtaining and analyzing the images are detailed in Supplemental Data 2. The retrospective analysis of human data and the waiver of informed consent were approved by the Institutional Review Board (2401-112-1505).
Statistical Analysis
All experiments were performed at least in triplicate, and data are presented as mean ± SD. Quantitative values were compared between groups using the Mann–Whitney U test (GraphPad Software version 9.5.1 for Microsoft Windows). All P values of less than 0.05 were considered to be significant.
RESULTS
Establishment of Hypertension Model
In the hypertension group, blood pressure was significantly higher than in the sham group (mean, 147.0 ± 27.9 vs. 111.4 ± 3.4 mm Hg; P = 0.004). Blood pressure in the ARB group was lower than that in the hypertension group (mean, 115.4 ± 8.3 mm Hg; P < 0.001). The hypertension group showed significantly lower body weight than the sham group from week 1 (19.48 ± 0.78 vs. 24.56 ± 1.24 g, P < 0.001), whereas the ARB group showed recovery (21.01 ± 0.82 g, P < 0.001). There was no significant difference in serum blood urea nitrogen level (25.48 ± 5.68, 29.23 ± 6.52, and 25.06 ± 4.09 mg/dL for the sham, hypertension, and ARB groups, respectively) or creatinine level (0.36 ± 0.03, 0.35 ± 0.04, and 0.37 ± 0.05 mg/dL for the sham, hypertension, and ARB groups, respectively) among the groups until week 4 after modeling (Supplemental Data 3).
68Ga-FAPI46 Uptake in Hypertension Model
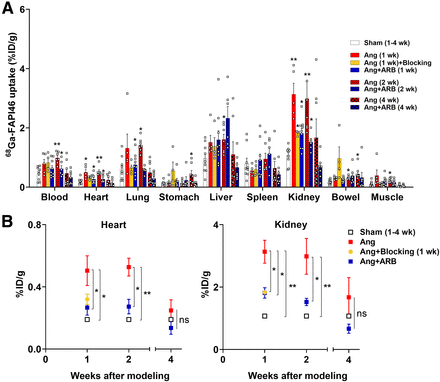
In the biodistribution study comparing the sham (n = 9) and the hypertensive animals, a mild increase in 68Ga-FAPI46 uptake was observed in the heart, which was significantly higher in the hypertension group (n = 5) than in the sham group at week 1 after modeling (0.50 ± 0.21 vs. 0.19 ± 0.10 %ID/g, P = 0.003). Uptake in the kidneys was prominently elevated in the hypertension group, compared with the sham group (3.13 ± 0.82 vs. 1.07 ± 0.30 %ID/g, P < 0.001), whereas no significant difference was observed in other organs (Fig. 1A). Uptake in the heart and kidneys was also elevated at week 2 (n = 7) but was decreased at week 4 (n = 7, Fig. 1B). Detailed data are shown in Supplemental Data 4.
Biodistribution study results. (A) Uptake in all target organs for each condition. (B) Serial changes in uptake in heart and kidneys. Data are presented as mean ± SEM. Angiotensin group was compared with sham group. ARB and blocking groups were compared with angiotensin group. *P < 0.05. **P < 0.001. Ang = angiotensin II; ns = not significant.
On 68Ga-FAPI46 PET/MRI, uptake in the heart and kidneys was similar to that on the biodistribution study. A mild increase in heart uptake and a prominent increase in kidney uptake were observed (Fig. 2). Uptake was higher in the hypertension group than in the sham group (n = 8) at week 1 (n = 10) and week 2 (n = 4) but was decreased at week 4 (n = 8). When the uptake of 68Ga-FAPI46 was quantitatively measured (Fig. 3), the TBRs of the heart and kidneys were significantly higher in the hypertension group than in the sham group, at week 1 (P < 0.001 for both) and week 2 (P = 0.008 and 0.004 for the heart and the kidneys, respectively). However, the difference was not observed at week 4 (Table 1). The SUVs demonstrated similar results and are presented in Supplemental Data 5.
68Ga-FAPI46 PET/MR images of sham and hypertension model mice at weeks 1, 2, and 4 after hypertension induction.
TBRs of heart (A) and kidneys (B) for each condition. Data are presented as mean ± SEM. Angiotensin group was compared with sham group. ARB and blocking groups were compared with angiotensin group. *P < 0.05. **P < 0.001. Ang = angiotensin II.
Quantitative Measurement of Uptake on 68Ga-FAPI46 PET/MRI
68Ga-FAPI46 Uptake in ARB Treatment and Blocking Study
ARB treatment reversed the increased uptake in the heart and kidneys observed in the hypertension group. The ARB group (n = 9, 6, and 7 for weeks 1, 2, and 4, respectively) exhibited significantly lower 68Ga-FAPI46 uptake in the heart and kidneys than did the hypertension group in the biodistribution study. The difference was consistently observed at weeks 1 and 2 (Fig. 1B). PET/MRI also revealed low uptake in the heart and kidneys in the ARB group (n = 10, 4, and 9 for weeks 1, 2, and 4, respectively; Fig. 4), with a significantly lower TBR than in the hypertension group (Table 1).
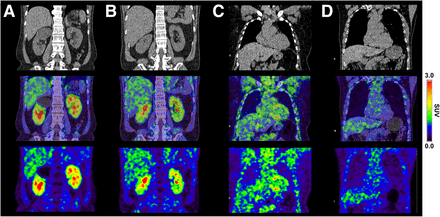
68Ga-FAPI46 PET/MR images of hypertension model mice receiving no treatment, high-dose blocking, and ARB treatment.
The blocking study at week 1 exhibited FAP-specific uptake of 68Ga-FAPI46 in the tissues. 68Ga-FAPI46 uptake in the heart and kidneys was lower in the blocking group than in the hypertension group in the biodistribution study (n = 4; Fig. 1), and this finding was also observed on PET/MRI (n = 5; Fig. 4) and TBR measurement (Table 1).
Histologic Changes and Protein Expression in Tissues
On Masson trichrome staining, myocardial tissue exhibited an evident fibrous portion in the left and right ventricular free walls as early as week 1 after hypertension induction. The fibrosis gradually progressed by week 4 (Fig. 5). Immunohistochemistry staining for vimentin showed similar results (Supplemental Data 6). FAP expression was also consistently observed on immunohistochemistry from weeks 1 through 4, in accordance with the biodistribution (Fig. 1B) and PET/MRI (Fig. 3). In contrast, renal tissue did not exhibit overt fibrosis on Masson trichrome staining at any time point. However, FAP expression was notably observed in the renal parenchyma, particularly at weeks 1 and 2.
Masson trichrome staining to assess fibrosis, and immunohistochemistry staining to assess FAP expression, in heart and kidney tissues. Scale bar is 500 μm.
Pilot Analysis of Human 68Ga-FAPI46 PET
Six patients (3 men and 3 women; age, 66 ± 3 y; range, 62–70 y) were in the hypertension group, and 10 patients (6 men and 4 women; age, 57 ± 12 y; range, 27–69 y) were in the control group. All patients exhibited normal renal function. In the hypertension group, the SUV and TBR were 1.88 ± 0.31 (median, 1.88; range, 1.39–2.37) and 2.20 ± 0.25 (median, 2.19; range, 1.91–2.58), respectively, which were higher than the 1.55 ± 0.21 (median, 1.47; range, 1.30–1.93) and 1.63 ± 0.23 (median, 1.64; range, 1.34–2.10) in the control group. The values differed significantly between the groups (P = 0.030 and 0.002, respectively), although the magnitude of the difference was small. Details on the results are provided in Supplemental Data 7. PET images of representative cases are shown in Figure 6.
68Ga-FAPI46 PET images of representative cases. (A) 64-y-old woman with hypertension. SUV and TBR of kidneys were 1.92 and 1.98, respectively. Renal cyst was also observed in right kidney upper pole. (B) 53-y-old man without hypertension. SUV and TBR of kidneys were 1.30 and 1.34, respectively. (C) 62-y-old woman with hypertension. Mildly increased uptake was observed in myocardium; SUV and TBR of heart were 3.10 and 3.23, respectively. (D) 69-y-old man without hypertension; SUV and TBR of heart were 1.47 and 1.56, respectively.
DISCUSSION
Fibrosis in CVD represents end-stage and irreversible tissue damage. In the cardiovascular system, fibrosis can lead to arteriosclerosis and myocardial distortion, with promotion of arrhythmia, cardiac remodeling, and heart failure (5,6,22,23). Renal interstitial fibrosis accelerates progression of chronic kidney disease by inducing glomerulosclerosis (9,10,24). Thus, assessment of tissue fibrosis could be a valuable tool in CVD management, by presenting disease status and treatment efficacy. Currently, strain analysis on echocardiography and late gadolinium enhancement on MRI are used for fibrosis imaging. However, these methods provide an indirect assessment of fibrosis and are limited to myocardial imaging.
The development of fibrosis in hypertension is associated with several pathophysiologic mechanisms. The renin–angiotensin system is known to be a key mediator in the regulation of blood pressure. Angiotensin II receptor type 1 is a G-protein–coupled receptor that plays a vital role in the renin–angiotensin system. Activation of angiotensin II receptor type 1 triggers the transforming growth factor-β1 signaling pathway, which results in excessive secretion of collagen and deposition of extracellular matrix, leading to fibrosis (5,8,9,24). Treatment targeting the renin–angiotensin system, such as angiotensin-converting enzyme inhibitors and ARBs, are expected to decelerate the progression of fibrosis, although there is limited supporting evidence.
FAP, a type II serine protease, is typically expressed on the cell surface of active fibroblasts and can also be shed in soluble form. FAP has both activity of postproline peptidase and activity of endopeptidase and cleaves proteins in adjacent tissues, facilitating proteolysis and remodeling of the extracellular matrix (25–27). FAP is prominently expressed on the surface of cancer-associated fibroblasts in various tumors (12), and several radiolabeled FAPIs are now being widely investigated as effective radiotracers for cancer imaging (12,15). Among them, FAPI46 presents a longer target retention than other FAPIs, making it a potentially valuable diagnostic and therapeutic agent (14,27,28).
FAP expression is increased in active benign fibrotic tissues, as well as in tumors. Recent studies have reported the application of FAPI PET in CVD and chronic kidney disease (16–20), with FAPI uptake being increased in infarcted myocardium and renal parenchyma, indicating activated tissue fibrosis in these conditions. In the present study, we applied FAPI PET to hypertension. In angiotensin II–induced hypertension, the renin–angiotensin system and related signaling pathways are activated, potentially involving the entire cardiovascular system in the pathogenesis (29). The heart and kidneys are the primary organs affected by hypertension, and clinical studies have demonstrated a high incidence of heart and renal failure in patients with chronic hypertension (30). We observed an early increase in 68Ga-FAPI46 uptake in the heart and kidneys, indicating that FAPI PET can detect early stages of fibrosis before overt manifestation.
Immunohistochemistry results exhibited increased FAP expression in both the heart and the kidneys. Intriguingly, overt fibrosis was observed in the myocardium using Masson trichrome staining, whereas it was not observed in renal tissue. The results suggest that FAP expression is not a simple surrogate marker for fibrosis. The progression of fibrosis in hypertension can be influenced by the characteristics of each organ. Hypertension imposes a direct pressure overload on the myocardium leading to ventricular hypertrophy, in which fibroblast proliferation and collagen deposition occur. It is speculated that FAP expression increases in the early phases of the process. Myocardial fibrosis is often a hemodynamic response and related to cardiac remodeling. Some recent studies have also reported FAP expression in the early phases of myocardial infarction and heart failure (17,20). In contrast, chronic hypertension may induce inflammation in the glomerular microvasculature and interstitium in the kidneys, leading to late fibrosis in the tissue repair process (31). Excessive accumulation of extracellular matrix affects both the function and the structure of the kidneys and causes renal failure (9,24,31). In our study, 68Ga-FAPI46 uptake was increased in renal tissues, without overt fibrosis and renal dysfunction.
To date, researchers investigating the impact of mechanical stress on the myocardium and blood vessels in hypertension have focused primarily on hemodynamic assessment. The present study suggests that FAPI PET can serve as a noninvasive imaging method to assess FAP expression and fibrosis activity, which can be a novel approach regarding the pathogenesis and progression of hypertension. Furthermore, FAPI PET may be effective in developing a treatment targeting fibrosis of major organ systems, as it has the potential to assess individual responses to the treatment.
This study had some limitations. First, we used the angiotensin II–induced hypertension model. Although this model has proven effective in many studies, it may not fully represent all types of hypertension and myocardial pressure overload. Further studies are needed to investigate the efficacy of 68Ga-FAPI46 PET in the assessment of tissue fibrosis in various CVD models. Second, uptake of 68Ga-FAPI46 in the target tissues was relatively low compared with the results of immunohistochemistry and Masson trichrome staining. This finding could be attributed to variations in FAPI46 affinity between humans and animals. Recent studies have reported that various FAPIs have lower affinities for murine FAP than for human FAP, despite their specificity for FAP (32,33). Additionally, the pilot human study involved 68Ga-FAPI46 PET images that were obtained without a controlled study design and protocols. Given that several FAPI PET agents are already used in human studies, it is feasible to design further studies using FAPI PET in human CVD. Finally, the functional parameters were not correlated with the FAPI uptake, which also needs to be investigated in further studies.
CONCLUSION
In the angiotensin II–induced hypertension model, FAP expression is increased in the heart and kidneys in the early phases and decreases over time in sustained hypertension. Overt fibrosis was observed in the heart but not in the kidneys over the course of 4 wk, suggesting that FAP expression can be a preceding or underlying marker for fibrosis. 68Ga-FAPI46 PET has the potential to be an effective imaging method for evaluating FAP expression in cardiac and renal tissues in progressive fibrosis caused by hypertension. 68Ga-FAPI46 PET can be a useful tool for evaluating CVDs and their treatment.
DISCLOSURE
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1277 to Jin Chul Paeng) and supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (RS-2024-00350443 to Jin Chul Paeng, NRF-2020R1A2C2006767 to Keon Wook Kang, and NRF-2020R1A2C2011428 to Gi Jeong Cheon). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 68Ga-FAPI46 PET feasible for early assessment of fibrosis in hypertension?
PERTINENT FINDINGS: In a hypertensive animal model, FAP expression was increased in the heart and kidneys. The FAPI uptake in the heart and kidney increased early after hypertension induction and gradually decreased, suggesting that FAP expression is an early response to hypertensive tissue change. In a preliminary human study, a trend toward increased FAPI uptake in the kidneys of hypertension patients was observed.
IMPLICATIONS FOR PATIENT CARE: It is warranted to test 68Ga-FAPI46 PET as an imaging modality for evaluating hypertension-induced changes, particularly in the kidneys.
Footnotes
Published online Sep. 26, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 31, 2024.
- Accepted for publication September 5, 2024.