Abstract
241346
Introduction: The IEC/NEMA PET phantom is widely used for acceptance testing and for comparison of scanner image quality. This phantom contains fillable spheres (diameter 10mm to 37mm) and a lung insert (containing foam beads) in a uniform background and is designed to simulate 18F-FDG PET body imaging and to evaluate lesion detection and contrast recovery. Prostate PSMA PET imaging, however, involves several factors not addressed well by the IEC/NEMA phantom. Often, the goal is to detect high-uptake lesions smaller than 10mm, including local recurrence near the bladder. Depending on the radiopharmaceutical, the bladder could have high activity concentration, which could cause artifacts and could affect detection and quantification of nearby lesions. A phantom that accurately represents prostate PET imaging would be highly valuable for evaluating the wide range of PET scanner models and clinical protocols in use.
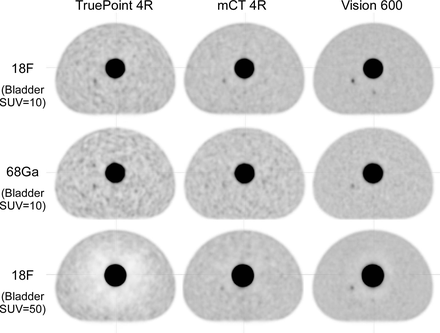
Methods: Simple modifications were made to an IEC/NEMA phantom to better evaluate prostate PET imaging. The existing cylindrical lung insert was instead designated as a hot bladder insert, which was emptied of foam beads and was filled with radioactive solution at 10:1 activity concentration versus background. The existing fillable spheres and mounting rods were removed. Acrylic rods were fabricated to mount three small fillable spheres (Data Spectrum Micro-Hollow Spheres) of diameter 4mm, 5mm, and 6.2mm in place of the existing spheres, placed below the bladder insert at the longitudinal center of the phantom and filled at 10:1 versus background. To compare a range of imaging performance, PET/CT scans of the phantom were acquired on three generations of Siemens Biograph scanners (TruePoint 4R, mCT 4R, and Vision 600). Listmode PET data were reconstructed over time intervals matching typical clinical prostate PET protocols for 18F-PSMA (9mCi injection, 60min uptake, 4min/bed) and 68Ga-PSMA (5mCi injection, 60min uptake, 5min/bed). Matching 18F scans also were acquired with the bladder insert at 50:1 and 1:1. Lesion detection was determined both by quantitative metric (SUVmax / (BkgSUVmax + BkgStdDev)) and by visual inspection.
Results: Lesion detection varied significantly between scanner models and clinical protocols. For the 18F clinical protocol, the 6mm sphere could be detected with high contrast (Vision) or with low contrast (mCT and TruePoint), and the 5mm sphere could be detected with low contrast only by the Vision. For the 68Ga clinical protocol, the 6mm sphere could be detected with low contrast only by the Vision. High bladder activity had strongest effect on the TruePoint (not time-of-flight (TOF) capable) and a noticeable effect on the mCT (lower TOF timing resolution than the Vision). SUV accuracy and presence of artifacts depended on scatter correction method (relative vs absolute) as well as TOF capability.
Conclusions: This modified IEC/NEMA phantom provides valuable insight regarding clinical prostate PET performance, considering the variety of available detector hardware, software, and radiopharmaceuticals. Evaluating small lesion detection and quantification accuracy with this phantom will help guide clinical protocols (radiopharmaceutical selection, dose, and scan time), optimization of image reconstruction parameters, and assessment of scanner technologies, including: detector crystals (resolution and scintillator type), ultra-fast TOF, larger axial field of view (total body PET), image reconstruction algorithms (including scatter and prompt-gamma corrections), and artificial intelligence image denoising.